Deck 12: Introduction to Organic Chemistry: Hydrocarbons
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/105
Play
Full screen (f)
Deck 12: Introduction to Organic Chemistry: Hydrocarbons
1
Which of the following is a condensed structural formula for an alkane with four carbon atoms?
A)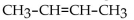
B)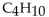
C)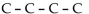
D)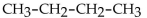
E)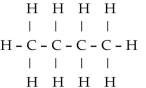
A)
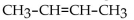
B)
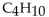
C)
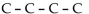
D)
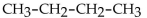
E)
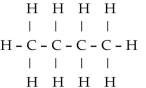
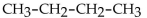
2
Organic chemistry is the study of the chemistry of compounds of
A)polymers.
B)oxygen.
C)hydrogen.
D)carbon.
E)living things.
A)polymers.
B)oxygen.
C)hydrogen.
D)carbon.
E)living things.
carbon.
3
How does a molecule of a vitamin synthesized in the laboratory behave when compared to the behavior of the same vitamin isolated from a natural source (e.g., vitamin C synthesized, compared to vitamin C from rose
Hips)?
A)Few effects are the same.
B)identical in every way
C)Some effects are the same.
D)usually identical
E)The natural vitamin is better.
Hips)?
A)Few effects are the same.
B)identical in every way
C)Some effects are the same.
D)usually identical
E)The natural vitamin is better.
identical in every way
4
In a molecule with a symmetrical arrangement of polar bonds, the overall molecule is
A)nonpolar.
B)reverse polar.
C)somewhat polar.
D)highly polar.
E)strongly reverse polar.
A)nonpolar.
B)reverse polar.
C)somewhat polar.
D)highly polar.
E)strongly reverse polar.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
5
Which of these formulas is an expanded structural formula for an alkane with three carbon atoms?
A)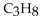
B)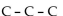
C)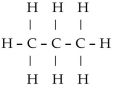
D)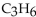
E)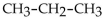
A)
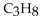
B)
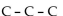
C)
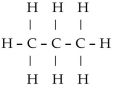
D)
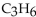
E)
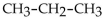

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
6
The bond angles of tetravalent carbon are all approximately
A)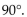
B)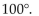
C)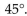
D)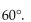
E)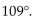
A)
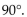
B)
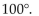
C)
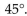
D)
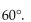
E)
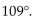

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
7
Carbon tetrachloride has a polar C-Cl bond. What is the overall polarity of the carbon tetrachloride molecule?
A)inverse polarity
B)weakly polar
C)strongly polar
D)nonpolar
E)reversed polarity
A)inverse polarity
B)weakly polar
C)strongly polar
D)nonpolar
E)reversed polarity

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
8
Generally, a solution of an organic compound in water will be electrically
A)highly conductive.
B)highly ionized.
C)insulated.
D)nonconductive.
E)charged.
A)highly conductive.
B)highly ionized.
C)insulated.
D)nonconductive.
E)charged.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
9
A molecule containing a carbon atom bonded to four chlorine atoms has the shape of a
A)square.
B)rhombus.
C)tetrahedron.
D)cube.
E)triangle.
A)square.
B)rhombus.
C)tetrahedron.
D)cube.
E)triangle.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
10
As carbon bonds with atoms of increasingly higher electronegativities, the polarity of the bond
A)decreases.
B)reverses.
C)becomes inverted.
D)increases.
E)stays the same.
A)decreases.
B)reverses.
C)becomes inverted.
D)increases.
E)stays the same.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
11
In a condensed structural formula, each carbon atom is
A)shown with all individual atoms and bonds drawn.
B)not explicitly shown.
C)shown with only the other carbon atoms.
D)written in lowercase letters.
E)grouped with its bonded hydrogen atoms.
A)shown with all individual atoms and bonds drawn.
B)not explicitly shown.
C)shown with only the other carbon atoms.
D)written in lowercase letters.
E)grouped with its bonded hydrogen atoms.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
12
How many valence electrons does carbon have?
A)one
B)two
C)three
D)four
E)five
A)one
B)two
C)three
D)four
E)five

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
13
VSEPR theory predicts that simple carbon compounds will form bonds that are
A)pointed to the corners of a triangle.
B)as far apart as possible.
C)as close together as possible.
D)arranged in a straight line.
E)pointed to the corners of a cube.
A)pointed to the corners of a triangle.
B)as far apart as possible.
C)as close together as possible.
D)arranged in a straight line.
E)pointed to the corners of a cube.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
14
Which one of the following is NOT an organic substance?
A)nylon
B)silk
C)salt, sodium chloride
D)coal
E)an antibiotic
A)nylon
B)silk
C)salt, sodium chloride
D)coal
E)an antibiotic

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is NOT typical of most organic compounds?
A)high melting point
B)covalent bonding
C)poor solubility in water
D)high flammability
E)low boiling point
A)high melting point
B)covalent bonding
C)poor solubility in water
D)high flammability
E)low boiling point

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
16
A formula that shows the arrangement of all bonds in a molecule is called a(n)
A)isomeric formula.
B)condensed structural formula.
C)molecular formula.
D)condensed molecular formula.
E)expanded structural formula.
A)isomeric formula.
B)condensed structural formula.
C)molecular formula.
D)condensed molecular formula.
E)expanded structural formula.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
17
A hydrocarbon contains only the elements
A)carbon and oxygen.
B)carbon, hydrogen, and oxygen.
C)hydrogen and oxygen.
D)carbon and hydrogen.
E)carbon, hydrogen, and nitrogen.
A)carbon and oxygen.
B)carbon, hydrogen, and oxygen.
C)hydrogen and oxygen.
D)carbon and hydrogen.
E)carbon, hydrogen, and nitrogen.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
18
Carbon atoms always have how many covalent bonds?
A)one
B)two
C)three
D)four
E)five
A)one
B)two
C)three
D)four
E)five

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
19
In the three-dimensional structure of methane, 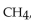 the hydrogen atoms attached to a carbon atom are aligned
the hydrogen atoms attached to a carbon atom are aligned
A) at the corners of a rectangle.
B) in a straight line.
C) at the corners of a cube.
D) at the corners of a square.
E) at the corners of a tetrahedron.
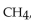 the hydrogen atoms attached to a carbon atom are aligned
the hydrogen atoms attached to a carbon atom are alignedA) at the corners of a rectangle.
B) in a straight line.
C) at the corners of a cube.
D) at the corners of a square.
E) at the corners of a tetrahedron.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
20
An organic compound composed of carbon and hydrogen connected only by single bonds is an
A)alkene.
B)alkyne.
C)alcohol.
D)alkane.
E)aromatic compound.
A)alkene.
B)alkyne.
C)alcohol.
D)alkane.
E)aromatic compound.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
21
What is the IUPAC name of this compound?
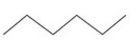
A) ethane
B) propane
C) pentane
D) hexane
E) butane

A) ethane
B) propane
C) pentane
D) hexane
E) butane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
22
What is the IUPAC name of this compound? 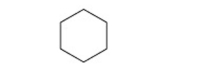
A)cyclopentane
B)hexane
C)cyclohexane
D)cycloheptane
E)cyclooctane

A)cyclopentane
B)hexane
C)cyclohexane
D)cycloheptane
E)cyclooctane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
23
What is the IUPAC name for a nine-carbon continuous-chain alkane?
A)hexane
B)heptane
C)octane
D)nonane
E)decane
A)hexane
B)heptane
C)octane
D)nonane
E)decane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
24
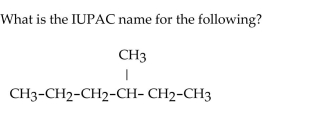
A)4-methylhexane
B)3-methylhexane
C)2-methylhexane
D)methylhexane
E)heptane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
25
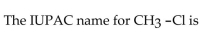
A)methanechlorine.
B)chloromethane.
C)methane chloride.
D)methyl chloride.
E)chloroethane.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
26
What is the IUPAC name for a four-carbon continuous-chain alkane?
A)ethane
B)propane
C)pentane
D)butane
E)methane
A)ethane
B)propane
C)pentane
D)butane
E)methane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
27
What is the IUPAC name for a six-carbon continuous-chain alkane?
A)hexane
B)heptane
C)octane
D)nonane
E)decane
A)hexane
B)heptane
C)octane
D)nonane
E)decane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
28
What is the IUPAC name for the following? 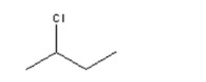
A)2-chlorobutane
B)3-chlorobutane
C)chlorobutane
D)2-chloropropane
E)1-chlorobutane

A)2-chlorobutane
B)3-chlorobutane
C)chlorobutane
D)2-chloropropane
E)1-chlorobutane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
29
What is the IUPAC name for a five-carbon continuous-chain alkane?
A)ethane
B)propane
C)pentane
D)methane
E)butane
A)ethane
B)propane
C)pentane
D)methane
E)butane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
30
What is the IUPAC name of this compound? 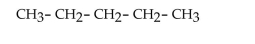
A)octane
B)heptane
C)hexane
D)methylbutane
E)pentane
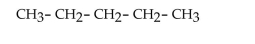
A)octane
B)heptane
C)hexane
D)methylbutane
E)pentane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following pairs of compounds are structural isomers?
A)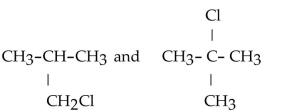
B)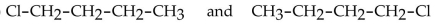
C)
D)
E)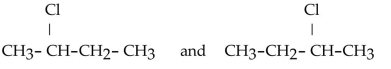
A)
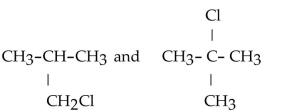
B)
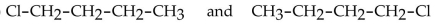
C)

D)

E)
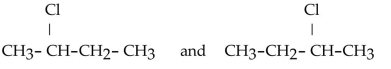

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
32
What is 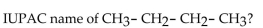
A)pentane
B)propane
C)ethane
D)butane
E)hexane
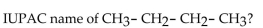
A)pentane
B)propane
C)ethane
D)butane
E)hexane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
33
What is the IUPAC name for 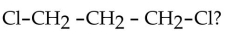
A) propane dichloride
B) 1,3-dichlorobutane
C) 1,1-dichloropropane
D) dichloropropane
E) 1,3 -dichloropropane
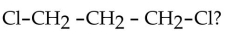
A) propane dichloride
B) 1,3-dichlorobutane
C) 1,1-dichloropropane
D) dichloropropane
E) 1,3 -dichloropropane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
34
What is the IUPAC name of this compound? 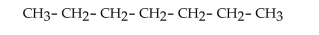
A)octane
B)heptane
C)butane
D)hexane
E)pentane
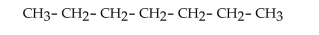
A)octane
B)heptane
C)butane
D)hexane
E)pentane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
35
What is the IUPAC name for a ten-carbon continuous-chain alkane?
A)hexane
B)heptane
C)octane
D)nonane
E)decane
A)hexane
B)heptane
C)octane
D)nonane
E)decane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
36
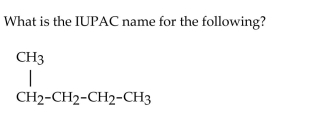
A)butane
B)1-methylbutane
C)4-methylbutane
D)hexane
E)pentane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
37
What is the IUPAC name for a seven-carbon continuous-chain alkane?
A)hexane
B)heptane
C)octane
D)nonane
E)decane
A)hexane
B)heptane
C)octane
D)nonane
E)decane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
38
What is the IUPAC name of the continuous chain alkane with six carbon atoms?
A)hexane
B)octane
C)heptane
D)butane
E)pentane
A)hexane
B)octane
C)heptane
D)butane
E)pentane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
39
What is the IUPAC name for an eight-carbon continuous-chain alkane?
A)hexane
B)heptane
C)octane
D)nonane
E)decane
A)hexane
B)heptane
C)octane
D)nonane
E)decane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
40
What is the IUPAC name of this compound? 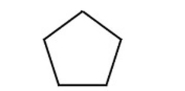
A)cyclohexane
B)cyclopentane
C)pentane
D)cycloheptane
E)cyclooctane

A)cyclohexane
B)cyclopentane
C)pentane
D)cycloheptane
E)cyclooctane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
41
In the equation for the combustion of pentane, the coefficient of carbon dioxide is
A)one
B)two.
C)three.
D)four.
E)five.
A)one
B)two.
C)three.
D)four.
E)five.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is true of nonane, 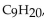 which has a density of 0.79 g/mL , melts at
which has a density of 0.79 g/mL , melts at 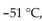 and boils at
and boils at 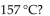
A)Nonane is soluble in water.
B)Nonane does not undergo combustion.
C)Nonane is a solid at room temperature.
D)Nonane is a gas at room temperature.
E)Nonane floats on the surface of water.
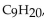 which has a density of 0.79 g/mL , melts at
which has a density of 0.79 g/mL , melts at 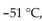 and boils at
and boils at 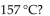
A)Nonane is soluble in water.
B)Nonane does not undergo combustion.
C)Nonane is a solid at room temperature.
D)Nonane is a gas at room temperature.
E)Nonane floats on the surface of water.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
43
What is the IUPAC name for this alkane? 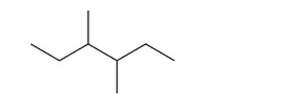
A)2, 3-diethylbutane
B)2-ethyl-3-methylpentane
C)4-ethyl-3-methylpentane
D)octane
E)3, 4-dimethylhexane

A)2, 3-diethylbutane
B)2-ethyl-3-methylpentane
C)4-ethyl-3-methylpentane
D)octane
E)3, 4-dimethylhexane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
44
What is the name of this compound? 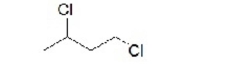
A)1,3-dichlorobutane
B)1,1-dichlorobutane
C)1,4-dichlorobutane
D)1,2,-dichlorobutane
E)dichlorobutane

A)1,3-dichlorobutane
B)1,1-dichlorobutane
C)1,4-dichlorobutane
D)1,2,-dichlorobutane
E)dichlorobutane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
45
The reaction for the combustion of heptane is 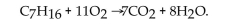 How many liters of
How many liters of 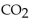 at STP are produced from the complete combustion of 2.00 moles of heptane?
at STP are produced from the complete combustion of 2.00 moles of heptane?
A) 44.8 L
B) 246 L
C) 22.4 L
D) 314 L
E) 157 L
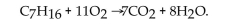 How many liters of
How many liters of 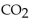 at STP are produced from the complete combustion of 2.00 moles of heptane?
at STP are produced from the complete combustion of 2.00 moles of heptane?A) 44.8 L
B) 246 L
C) 22.4 L
D) 314 L
E) 157 L

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
46
When drawing a structural formula, the first step is to draw
A)the substituents.
B)the main carbon chain.
C)the functional group.
D)the saturated carbons.
E)the most highly substituted carbons.
A)the substituents.
B)the main carbon chain.
C)the functional group.
D)the saturated carbons.
E)the most highly substituted carbons.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following compounds could have the molecular formula C7H16?
A)pentane
B)hexane
C)2-methylheptane
D)3-ethylhexane
E)2,3-dimethylpentane
A)pentane
B)hexane
C)2-methylheptane
D)3-ethylhexane
E)2,3-dimethylpentane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
48
According to the IUPAC convention, alkyl group names should be located ________ of the name of the main chain.
A)at the end
B)in front
C)in the middle
A)at the end
B)in front
C)in the middle

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
49
What is the IUPAC name for the following? 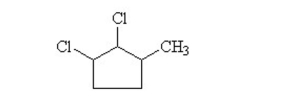
A)2,3-dichloro-1-methylcyclopentane
B)2,3-dichloro-1-methylpentane
C)1,2-dichloro-3-methylcyclobutane
D)3-methyl-1,2-dichlorocyclopentane
E)1-methyl-2,3-dichlorocyclopentane

A)2,3-dichloro-1-methylcyclopentane
B)2,3-dichloro-1-methylpentane
C)1,2-dichloro-3-methylcyclobutane
D)3-methyl-1,2-dichlorocyclopentane
E)1-methyl-2,3-dichlorocyclopentane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
50
What is the IUPAC name for this three-carbon alkyl group? 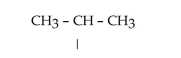
A)iso-propyl
B)ethyl
C)butyl
D)iso-butyl
E)n-propyl
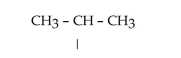
A)iso-propyl
B)ethyl
C)butyl
D)iso-butyl
E)n-propyl

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
51
What is the name for the following? 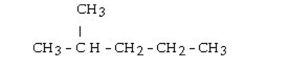
A)pentane
B)4-methylpentane
C)2-methylpentane
D)methylpentane
E)hexane
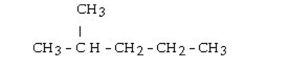
A)pentane
B)4-methylpentane
C)2-methylpentane
D)methylpentane
E)hexane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
52
What is the IUPAC name of this alkane? 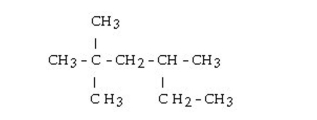
A)2-ethyl-4,4-dimethylpentane
B)4-ethyl-2,2-dimethylpentane
C)3,5,5-trimethylhexane
D)2-ethyl-2,2-dimethylpentane
E)2,2,4-trimethylhexane

A)2-ethyl-4,4-dimethylpentane
B)4-ethyl-2,2-dimethylpentane
C)3,5,5-trimethylhexane
D)2-ethyl-2,2-dimethylpentane
E)2,2,4-trimethylhexane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
53
According to the IUPAC convention for chemical naming, which part of a hydrocarbon is selected as the main chain for a hydrocarbon chain?
A)the chain with the most substituted carbons in it
B)the most highly branched chain
C)the longest chain drawn in a straight line
D)the shortest chain
E)the longest continuous chain, regardless of bends
A)the chain with the most substituted carbons in it
B)the most highly branched chain
C)the longest chain drawn in a straight line
D)the shortest chain
E)the longest continuous chain, regardless of bends

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
54
What is the IUPAC name for a three-carbon alkyl group?
A)methyl
B)ethyl
C)propyl
D)butyl
E)pentyl
A)methyl
B)ethyl
C)propyl
D)butyl
E)pentyl

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
55
Organic compounds that are poorly soluble in water behave that way because they are
A)covalently bonded.
B)generally nonpolar.
C)moderately polar.
D)highly polar.
E)ionically bonded.
A)covalently bonded.
B)generally nonpolar.
C)moderately polar.
D)highly polar.
E)ionically bonded.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
56
What is the IUPAC name for a two-carbon alkyl group?
A)methyl
B)ethyl
C)propyl
D)butyl
E)pentyl
A)methyl
B)ethyl
C)propyl
D)butyl
E)pentyl

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
57
What is the name of the alkyl group 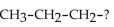
A) ethane
B) ethyl
C) propane
D) methyl
E) propyl
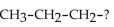
A) ethane
B) ethyl
C) propane
D) methyl
E) propyl

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
58
According to the IUPAC convention, alkyl substituents on a hydrocarbon chain should be listed in which order?
A)in order with the substituent having the lowest number of carbons first
B)alphabetical without considering prefixes
C)in order with the substituent having the highest total number of carbons first
D)alphabetical including prefixes
E)in order with the substituent having the highest number of carbons first
A)in order with the substituent having the lowest number of carbons first
B)alphabetical without considering prefixes
C)in order with the substituent having the highest total number of carbons first
D)alphabetical including prefixes
E)in order with the substituent having the highest number of carbons first

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
59
What is the IUPAC name for a one-carbon alkyl substituent?
A)methyl
B)ethyl
C)propyl
D)butyl
E)pentyl
A)methyl
B)ethyl
C)propyl
D)butyl
E)pentyl

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
60
What is the name for the following? 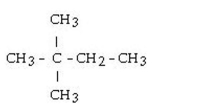
A)dimethylbutane
B)hexane
C)2-dimethylbutane
D)2,2-dimethylbutane
E)3,3-dimethylbutane

A)dimethylbutane
B)hexane
C)2-dimethylbutane
D)2,2-dimethylbutane
E)3,3-dimethylbutane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
61
A hydrocarbon with at least one double bond is a(n)
A)alcohol.
B)alkyne.
C)saturated compound.
D)alkene.
E)alkane.
A)alcohol.
B)alkyne.
C)saturated compound.
D)alkene.
E)alkane.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
62
The IUPAC name for ethylene is
A)ethanene.
B)ethane.
C)cycloethane.
D)ethene.
E)ethyne.
A)ethanene.
B)ethane.
C)cycloethane.
D)ethene.
E)ethyne.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
63
The IUPAC name for 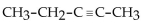 is
is
A) pentyne.
B) 3 -pentyne.
C) 1-methylbutyne.
D) 2-pentyne.
E) 2-propene.
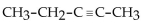 is
isA) pentyne.
B) 3 -pentyne.
C) 1-methylbutyne.
D) 2-pentyne.
E) 2-propene.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
64
What type of compound is 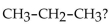
A) branched alkane
B) haloalkane
C) isomer
D) alkane
E) cycloalkane
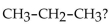
A) branched alkane
B) haloalkane
C) isomer
D) alkane
E) cycloalkane

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
65
What is the condensed structural formula for the compound 3-hexene?
A)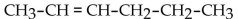
B)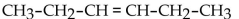
C)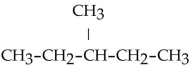
D)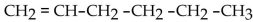
E)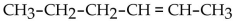
A)
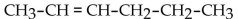
B)
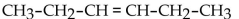
C)
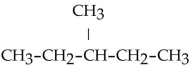
D)
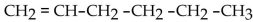
E)
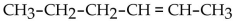

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following is a property of nonane, 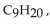 which has a density of 0.79 g/mL, melts at
which has a density of 0.79 g/mL, melts at 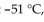 and boils at
and boils at 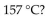
A) Nonane does not undergo combustion.
B) Nonane solutions conduct electricity.
C) Nonane is not soluble in water.
D) Nonane is a gas at room temperature.
E) Nonane sinks in water.
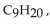 which has a density of 0.79 g/mL, melts at
which has a density of 0.79 g/mL, melts at 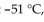 and boils at
and boils at 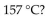
A) Nonane does not undergo combustion.
B) Nonane solutions conduct electricity.
C) Nonane is not soluble in water.
D) Nonane is a gas at room temperature.
E) Nonane sinks in water.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following compounds is an alkyne?
A)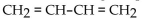
B)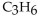
C) 2 -pentene
D)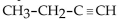
E)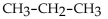
A)
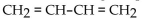
B)
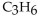
C) 2 -pentene
D)
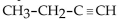
E)
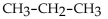

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
68
The reaction of butane with oxygen is called
A)substitution.
B)combustion.
C)addition.
D)neutralization.
E)titration.
A)substitution.
B)combustion.
C)addition.
D)neutralization.
E)titration.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
69
The carbon atoms in saturated hydrocarbons
A)contain a benzene ring.
B)contain both a double and a triple bond.
C)contain at least one triple bond.
D)have only single bonds.
E)contain at least one double bond.
A)contain a benzene ring.
B)contain both a double and a triple bond.
C)contain at least one triple bond.
D)have only single bonds.
E)contain at least one double bond.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
70
An alkene is a hydrocarbon that contains at least one ________ bond.
A)triple
B)aromatic
C)single
D)double
E)hydrogen
A)triple
B)aromatic
C)single
D)double
E)hydrogen

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
71
The compound 1-butyne contains
A)a ring structure.
B)all single bonds.
C)a triple bond.
D)a double bond.
E)a bromine atom.
A)a ring structure.
B)all single bonds.
C)a triple bond.
D)a double bond.
E)a bromine atom.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
72
Hydrocarbons are the primary constituents of
A)drugs.
B)food flavors.
C)gasoline.
D)disinfectants.
E)fruit juices.
A)drugs.
B)food flavors.
C)gasoline.
D)disinfectants.
E)fruit juices.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
73
The IUPAC name of 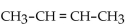 is
is
A) 1-butene.
B) 2-butyne.
C) 2-butene.
D) butene.
E) 2-butane.
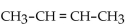 is
isA) 1-butene.
B) 2-butyne.
C) 2-butene.
D) butene.
E) 2-butane.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the compounds is a cycloalkene?
A)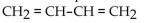
B)
C)
D)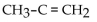
E)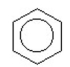
A)
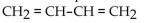
B)

C)

D)
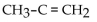
E)


Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
75
What is the condensed structural formula of the compound propene?
A)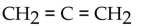
B)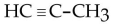
C)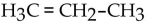
D)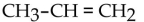
E)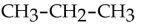
A)
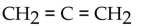
B)
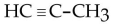
C)
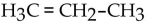
D)
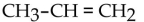
E)
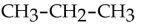

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
76
When naming an alkene, the parent chain is the longest carbon chain
A)that contains at least one of the carbon atoms of the double bond.
B)that does not contain the double bond.
C)regardless of whether or not it contains the double bond.
D)that contains the double bond.
E)that contains a branch.
A)that contains at least one of the carbon atoms of the double bond.
B)that does not contain the double bond.
C)regardless of whether or not it contains the double bond.
D)that contains the double bond.
E)that contains a branch.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
77
The balanced equation for the complete combustion of 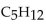 will give which of these product(s)?
will give which of these product(s)?
A)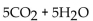
B)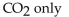
C)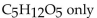
D)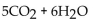
E) 10CO + 12H2O
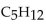 will give which of these product(s)?
will give which of these product(s)?A)
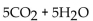
B)
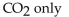
C)
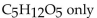
D)
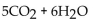
E) 10CO + 12H2O

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
78
An unsaturated compound always
A)is a cycloalkane.
B)contains a double bond.
C)contains at least one double or triple bond.
D)contains a triple bond.
E)is aromatic.
A)is a cycloalkane.
B)contains a double bond.
C)contains at least one double or triple bond.
D)contains a triple bond.
E)is aromatic.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
79
What is(are) the product(s) of the complete combustion of any hydrocarbon?
A) CO only
B)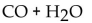
C)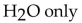
D)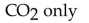
E)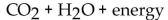
A) CO only
B)
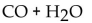
C)
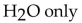
D)
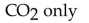
E)
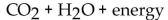

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
80
An alkyne is a hydrocarbon that contains at least one________ bond.
A)triple
B)aromatic
C)hydrogen
D)single
E)double
A)triple
B)aromatic
C)hydrogen
D)single
E)double

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck


