Deck 22: The Second Law of Thermodynamics, Heat Engines and Entropy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/43
Play
Full screen (f)
Deck 22: The Second Law of Thermodynamics, Heat Engines and Entropy
1
A heat pump has a coefficient of performance of 4. If the heat pump absorbs 20 kJ of heat from the cold outdoors in each cycle, the heat expelled (in kJ) to the warm indoors is:
A)34
B)27
C)36
D)40
E)80
A)34
B)27
C)36
D)40
E)80
27
2
A heat engine absorbs 2500 J of heat from a hot reservoir and expels 1000 J to a cold reservoir. When it is run in reverse, with the same reservoirs, the engine pumps 2500 J of heat to the hot reservoir, requiring 1500 J of work to do so. Find the ratio of the work done by the heat engine to the work done by the pump. Is the heat engine reversible?
A)1.0 (no)
B)1.0 (yes)
C)1.5 (yes)
D)1.5 (no)
E)2.5 (no)
A)1.0 (no)
B)1.0 (yes)
C)1.5 (yes)
D)1.5 (no)
E)2.5 (no)
1.0 (yes)
3
A company that produces pulsed gas heaters claims their efficiency is approximately 90%. If an engine operates between 250 C and 25 C, what is its maximum thermodynamic efficiency?
A)83%
B)65%
C)43%
D)90%
E)56%
A)83%
B)65%
C)43%
D)90%
E)56%
43%
4
An ideal gas is allowed to expand adiabatically. Assume the process is reversible. The change in entropy is:
A)0
B)nR ln (V2/V1)
C)nR ln (T2/T1)
D)kn ln (V2/V1)
E)kn ln (T2/T1)
A)0
B)nR ln (V2/V1)
C)nR ln (T2/T1)
D)kn ln (V2/V1)
E)kn ln (T2/T1)

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
5
When water of mass m and specific heat c is heated from absolute temperature T1 to absolute temperature T2, its change in entropy is:
A)cm ln(T2/T1)
B)cm (T2/T1)
C)cm (T2 - T1)/T1
D)cm (T2 - T1)/T2
E)cm (T2 - T1)/(T2 + T1)
A)cm ln(T2/T1)
B)cm (T2/T1)
C)cm (T2 - T1)/T1
D)cm (T2 - T1)/T2
E)cm (T2 - T1)/(T2 + T1)

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
6
An ideal gas is allowed to undergo a free expansion. If its initial volume is V1 and its final volume is V2, the change in entropy is:
A)nR ln (V2/V1)
B)nRT ln (V2/V1)
C)nk ln (V2/V1)
D)0
E)nR V2/V1
A)nR ln (V2/V1)
B)nRT ln (V2/V1)
C)nk ln (V2/V1)
D)0
E)nR V2/V1

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
7
A gasoline engine absorbs 2500 J of heat and performs 1000 J of mechanical work in each cycle. The efficiency of the engine is:
A)80%
B)40%
C)60%
D)20%
E)50%
A)80%
B)40%
C)60%
D)20%
E)50%

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
8
Determine the change in entropy (in J/K) when 5.00 moles of an ideal gas at 0 C are compressed isothermally from an initial volume of 100 cm3 to a final volume of 20 cm3.
A)(-191)
B)(-52)
C)(-71)
D)(-67)
E)(-208)
A)(-191)
B)(-52)
C)(-71)
D)(-67)
E)(-208)

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
9
A gasoline engine absorbs 2500 J of heat and performs 1000 J of mechanical work in each cycle. The amount of heat expelled in each cycle is:
A)1000 J
B)1500 J
C)2000 J
D)500 J
E)3000 J
A)1000 J
B)1500 J
C)2000 J
D)500 J
E)3000 J

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
10
Exactly 500 grams of ice are melted at a temperature of 0 C. (Lice = 333 J/g.) The change in entropy (in J/K) is:
A)321
B)146
C)512
D)610
E)5230
A)321
B)146
C)512
D)610
E)5230

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
11
If n moles of an ideal gas are compressed isothermally from an initial volume V1 to a final volume V2, the change in entropy is:
A)nR ln (V2/V1)
B)nRT ln (V2/V1)
C)nkB ln (V2/V1)
D)
E)n Cv/T
A)nR ln (V2/V1)
B)nRT ln (V2/V1)
C)nkB ln (V2/V1)
D)
E)n Cv/T

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
12
An automobile engine operates with an overall efficiency of 12%. How much energy is delivered as waste heat (in litres of petrol) for each 10 litres of petrol burned?
A)1.2
B)8.8
C)6.5
D)4.7
E)7.5
A)1.2
B)8.8
C)6.5
D)4.7
E)7.5

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
13
A new electric power plant has an efficiency of 42%. For every 100 barrels of oil needed to run the turbine, how many are essentially lost as waste heat (in barrels of oil) to the environment?
A)21
B)42
C)58
D)10
E)79
A)21
B)42
C)58
D)10
E)79

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
14
Find the change in entropy (in J/K) when 5.00 moles of an ideal gas undergo a free expansion from an initial volume of 20 cm3 to a final volume of 100 cm3.
A)71
B)52
C)67
D)191
E)208
A)71
B)52
C)67
D)191
E)208

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
15
An engine is designed to obtain energy from the temperature gradient of the ocean. What is the thermodynamic efficiency of such an engine if the temperature of the surface of the water is 15 C and the temperature well below the surface is 5 C?
A)3.5%
B)67%
C)31%
D)17%
E)96%
A)3.5%
B)67%
C)31%
D)17%
E)96%

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
16
An 800-MW electric power plant has an efficiency of 30%. It loses its waste heat in large cooling towers. Approximately how much waste heat (in MJ) is discharged to the atmosphere per second?
A)1200
B)1900
C)800
D)560
E)240
A)1200
B)1900
C)800
D)560
E)240

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
17
A vessel containing 5.0 kg of water at 10 C is put into a refrigerator. The 1/7 HP motor (1 HP = 746 W) runs for 5.0 minutes to cool the liquid to the refrigerator's low temperature, 0 C. What is the COP of the refrigerator?
A)5.7
B)4.6
C)6.5
D)7.2
E)3.6
A)5.7
B)4.6
C)6.5
D)7.2
E)3.6

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
18
A lawn mower has a 6-horsepower engine (1 HP = 750 W). If the engine has an efficiency of 20%, and the throttle is such that the engine cycles 10 times a second, the heat that is expelled in one cycle is:
A)1800 J
B)2000 J
C)2200 J
D)2400 J
E)2250 J
A)1800 J
B)2000 J
C)2200 J
D)2400 J
E)2250 J

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
19
The change in entropy of a mass m of a solid substance which has a latent heat of fusion L and melts at a temperature T is:
A)LT/m
B)mL ln(T)
C)mLT
D)mL/T
E)L/mT
A)LT/m
B)mL ln(T)
C)mLT
D)mL/T
E)L/mT

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
20
A steam engine is operating at its theoretical maximum efficiency of 60%. If the waste heat has a temperature of 38 C, what is the temperature of the boiler?
A)350 C
B)94 C
C)225 C
D)504 C
E)775 C
A)350 C
B)94 C
C)225 C
D)504 C
E)775 C

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is an almost reversible process?
A)The explosion of hydrogen and oxygen to form water.
B)Heat transfer through thick insulation.
C)The adiabatic free expansion of a gas.
D)A slow isothermal compression of a gas.
E)A slow leakage of gas into an empty chamber through a small hole in a membrane.
A)The explosion of hydrogen and oxygen to form water.
B)Heat transfer through thick insulation.
C)The adiabatic free expansion of a gas.
D)A slow isothermal compression of a gas.
E)A slow leakage of gas into an empty chamber through a small hole in a membrane.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
22
The reason that we can calculate the change in entropy of a system is that:
A)entropy always decreases.
B)entropy always increases.
C)the entropy of the universe always remains constant.
D)it depends only on the properties of the initial and final equilibrium states.
E)systems always follow reversible paths.
A)entropy always decreases.
B)entropy always increases.
C)the entropy of the universe always remains constant.
D)it depends only on the properties of the initial and final equilibrium states.
E)systems always follow reversible paths.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
23
An ideal heat engine can have an efficiency of 1 if the temperature of the low temperature reservoir is:
A)0 K.
B)0 C.
C)0 F.
D)0 R.
E)the same as the temperature of the heat source.
A)0 K.
B)0 C.
C)0 F.
D)0 R.
E)the same as the temperature of the heat source.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
24
Suppose there are 3 molecules in a container. If each molecule has a 1-in-2 chance of being in the left half of the container, what is the probability that there are exactly 2 molecules in the left half of the container?
A)1/2
B)1/3
C)1/8
D)3/8
E)3/5
A)1/2
B)1/3
C)1/8
D)3/8
E)3/5

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
25
A violation of the second law of thermodynamics would occur if:
A)a ball lying on the ground started to bounce.
B)transfer of energy by heat moved energy from an object at low temperature to an object at a higher temperature.
C)a refrigerator heated the air in the room in which the refrigerator is located.
D)any of the above occurred.
E)(a) or (b) occurred, but not (c).
A)a ball lying on the ground started to bounce.
B)transfer of energy by heat moved energy from an object at low temperature to an object at a higher temperature.
C)a refrigerator heated the air in the room in which the refrigerator is located.
D)any of the above occurred.
E)(a) or (b) occurred, but not (c).

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
26
For the same temperature increase in a system, the change in entropy, S, is largest in a reversible:
A)constant volume process.
B)constant pressure process.
C)adiabatic process.
D)process in which no heat is transferred.
E)process in which no work is performed.
A)constant volume process.
B)constant pressure process.
C)adiabatic process.
D)process in which no heat is transferred.
E)process in which no work is performed.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
27
Which answer below is not a statement of the second law of thermodynamics?
A)Real processes proceed in a preferred direction.
B)Energy does not flow spontaneously by heat from a cold to a hot reservoir.
C)The entropy of the universe increases in all natural processes.
D)In theory, heat engines working in a cycle employ reversible processes.
E)You cannot construct a heat engine operating in a cycle that does nothing but take heat from a reservoir and perform an equal amount of work.
A)Real processes proceed in a preferred direction.
B)Energy does not flow spontaneously by heat from a cold to a hot reservoir.
C)The entropy of the universe increases in all natural processes.
D)In theory, heat engines working in a cycle employ reversible processes.
E)You cannot construct a heat engine operating in a cycle that does nothing but take heat from a reservoir and perform an equal amount of work.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
28
For a gas of N identical molecules of molecular volume Vm in total volume V at temperature T, the number of ways of locating the N molecules in the volume is:
A) .
B) .
C) .
D) .
E) .
A) .
B) .
C) .
D) .
E) .

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
29
A petrol engine absorbs 2500 J of heat at 250 C and expels 2000 J at a temperature of 50 C. The magnitude of the change in entropy (in J/K) for the system is:
A)6.2
B)4.7
C)1.4
D)10.9
E)3.2
A)6.2
B)4.7
C)1.4
D)10.9
E)3.2

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
30
An adiabatic free expansion of a gas in a thermally isolated container is not reversible because:
A)work must be done on the gas to return it to its original volume.
B)heat must be exchanged with the surroundings to return the gas to its original temperature.
C)its internal energy has a greater value after the expanded gas is returned to its original volume and temperature.
D)of all of the above.
E)of (a) and (b) above only.
A)work must be done on the gas to return it to its original volume.
B)heat must be exchanged with the surroundings to return the gas to its original temperature.
C)its internal energy has a greater value after the expanded gas is returned to its original volume and temperature.
D)of all of the above.
E)of (a) and (b) above only.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
31
A Carnot cycle, operating as a heat engine, consists, in the order given, of:
A)an isothermal expansion, an isothermal compression, an adiabatic expansion and an adiabatic compression.
B)an adiabatic expansion, an adiabatic compression, an isothermal expansion and an isothermal compression.
C)an isothermal expansion, an adiabatic compression, an isothermal compression and an adiabatic expansion.
D)an adiabatic compression, an isothermal compression, an isothermal expansion and an adiabatic expansion.
E)an isothermal expansion, an adiabatic expansion, an isothermal compression and an adiabatic compression.
A)an isothermal expansion, an isothermal compression, an adiabatic expansion and an adiabatic compression.
B)an adiabatic expansion, an adiabatic compression, an isothermal expansion and an isothermal compression.
C)an isothermal expansion, an adiabatic compression, an isothermal compression and an adiabatic expansion.
D)an adiabatic compression, an isothermal compression, an isothermal expansion and an adiabatic expansion.
E)an isothermal expansion, an adiabatic expansion, an isothermal compression and an adiabatic compression.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
32
By operating a reversible heat engine with an ideal gas as the working substance in a Carnot cycle and measuring the ratio Qc/Qh, we can calculate:
A)n, the number of moles of the ideal gas.
B)the ratio Vc/Vh of the volumes of the ideal gas.
C)the ratio Pc/Ph of the pressures of the ideal gas.
D)the ratio PcVc/PhVh of the products of volumes and pressures of the ideal gas.
E)the value of Avogadro's number.
A)n, the number of moles of the ideal gas.
B)the ratio Vc/Vh of the volumes of the ideal gas.
C)the ratio Pc/Ph of the pressures of the ideal gas.
D)the ratio PcVc/PhVh of the products of volumes and pressures of the ideal gas.
E)the value of Avogadro's number.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
33
A violation of the second law of thermodynamics would occur if:
A)a ball lying on the ground started to bounce.
B)transfer of energy by heat moved energy from an object at low temperature to an object at a higher temperature.
C)a motion picture was run backwards through the projector.
D)any of the above occurred.
E)(a) or (b) occurred, but not (c).
A)a ball lying on the ground started to bounce.
B)transfer of energy by heat moved energy from an object at low temperature to an object at a higher temperature.
C)a motion picture was run backwards through the projector.
D)any of the above occurred.
E)(a) or (b) occurred, but not (c).

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
34
Find the change in entropy (in J/K) when 5.00 moles of an ideal monatomic gas are allowed to expand isobarically from an initial volume of 20 cm3 to a final volume of 100 cm3.
A)167
B)100
C)67
D)52
E)152
A)167
B)100
C)67
D)52
E)152

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
35
Selena states that 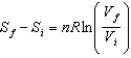 proves that entropy has a definite value at the beginning and end of an adiabatic free expansion. Ron says
proves that entropy has a definite value at the beginning and end of an adiabatic free expansion. Ron says 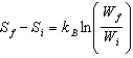 , where W is the number of microstates of a given macrostate. Which one, if either, is correct?
, where W is the number of microstates of a given macrostate. Which one, if either, is correct?
A)Only Selena, because entropy can depend only on macroscopic variables.
B)Only Ron, because entropy can depend only on microscopic variables.
C)Only Selena, because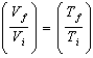 in an adiabatic free expansion.
in an adiabatic free expansion.
D)Neither, because we cannot calculate changes in entropy in an adiabatic free expansion.
E)Both, because entropy, which is macroscopic, is a function of microscopic disorder.
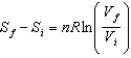 proves that entropy has a definite value at the beginning and end of an adiabatic free expansion. Ron says
proves that entropy has a definite value at the beginning and end of an adiabatic free expansion. Ron says 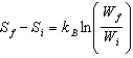 , where W is the number of microstates of a given macrostate. Which one, if either, is correct?
, where W is the number of microstates of a given macrostate. Which one, if either, is correct?A)Only Selena, because entropy can depend only on macroscopic variables.
B)Only Ron, because entropy can depend only on microscopic variables.
C)Only Selena, because
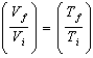 in an adiabatic free expansion.
in an adiabatic free expansion.D)Neither, because we cannot calculate changes in entropy in an adiabatic free expansion.
E)Both, because entropy, which is macroscopic, is a function of microscopic disorder.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
36
For a pair of dice, how many microstates are there that result in the macrostate of rolling a 7?
A)1
B)5
C)6
D)7
E)36
A)1
B)5
C)6
D)7
E)36

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
37
All real engines are less efficient than the Carnot engine because:
A)they do not operate through the Otto cycle.
B)they do not operate through a reversible cycle.
C)the working substance does not maintain a constant volume through the cycle.
D)the working substance does not maintain a constant pressure through the cycle.
E)the working substance does not maintain a constant temperature through the cycle.
A)they do not operate through the Otto cycle.
B)they do not operate through a reversible cycle.
C)the working substance does not maintain a constant volume through the cycle.
D)the working substance does not maintain a constant pressure through the cycle.
E)the working substance does not maintain a constant temperature through the cycle.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
38
A 4.00-litre container is divided into two equal parts, one part containing 0.010 0 mole of neon, and the other part containing 0.010 0 mole of helium. The divider is removed and the gases mix. What is the change in entropy (in J/K) of this system?
A)0.057 6
B)0.115
C)0.173
D)0.086 4
E)0.144
A)0.057 6
B)0.115
C)0.173
D)0.086 4
E)0.144

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
39
The thermal efficiency of a heat engine is given by:
A)
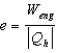 .
.B)
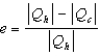 .
.C)
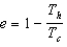 .
.D)all of the formulas above.
E)only (a) or (b) above.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
40
What is the probability of rolling either a 7 or 11 on a single roll of a pair of dice?
A)1/6
B)2/9
C)3/8
D)1/3
E)1/2
A)1/6
B)2/9
C)3/8
D)1/3
E)1/2

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
41
In a nuclear power plant, the reactor produces 500 C steam that is used to power the steam turbines which generate 1500 MW of electrical power. The cooling tower eliminates the waste heat at 50 C. If the efficiency of the plant were that of a Carnot engine, at what rate would waste heat be vented to the atmosphere?

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
42
One end of a copper rod is in thermal contact with a hot reservoir at T = 500 K and the other end is in thermal contact with a cooler reservoir at T = 300 K. Find the entropy change of each reservoir, and the total entropy change, when 8000 J of heat energy are conducted from one end of the rod to the other with no change in the temperature distribution in the rod.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
43
Every second at Niagara Falls, some 5000 m3 of water falls a distance of 50 m. What is the increase in entropy per second due to the falling water? (Assume a 20 C environment).

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck


