Deck 21: The Kinetic Theory of Gases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/35
Play
Full screen (f)
Deck 21: The Kinetic Theory of Gases
1
A molecule in a uniform ideal gas can collide with other molecules when their centres are equal to or less than:
A)one radius away from its centre.
B)one diameter away from its centre.
C)two diameters away from its centre.
D)twice the cube root of volume away from its centre.
E) diameters away from its centre.
A)one radius away from its centre.
B)one diameter away from its centre.
C)two diameters away from its centre.
D)twice the cube root of volume away from its centre.
E) diameters away from its centre.
B
2
The relation PV = nRT holds for all ideal gases. The additional relation PV holds for an adiabatic process. The figure below shows two curves: one is an adiabat and one is an isotherm. Each starts at the same pressure and volume. Which statement is correct? (Note: ' ' means 'is proportional to'.) 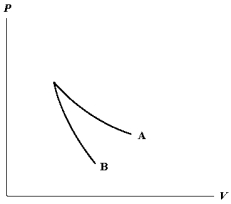
A)Isotherm: ; Adiabat:
: A is both an isotherm and an adiabat.
B)Isotherm: ; Adiabat:
: B is an isotherm, A is an adiabat.
C)Isotherm: ; Adiabat:
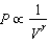 : A is an isotherm, B is an adiabat.
: A is an isotherm, B is an adiabat.
D)Isotherm: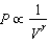 ; Adiabat:
; Adiabat:
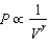 : B is both an isotherm and an adiabat.
: B is both an isotherm and an adiabat.
E)cannot answer without additional information about the starting temperature.

A)Isotherm: ; Adiabat:
: A is both an isotherm and an adiabat.
B)Isotherm: ; Adiabat:
: B is an isotherm, A is an adiabat.
C)Isotherm: ; Adiabat:
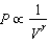 : A is an isotherm, B is an adiabat.
: A is an isotherm, B is an adiabat.D)Isotherm:
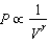 ; Adiabat:
; Adiabat: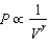 : B is both an isotherm and an adiabat.
: B is both an isotherm and an adiabat.E)cannot answer without additional information about the starting temperature.
C
3
When we say that the speed of sound is measured under adiabatic conditions we assume that:
A)the time associated with heat conduction is slow relative to the speed of the wave.
B)no heat can flow between the system and its surroundings.
C)the speed of the wave is directly proportional to the bulk modulus.
D)the speed of the wave is proportional to the square root of the bulk modulus.
E)air is an ideal gas.
A)the time associated with heat conduction is slow relative to the speed of the wave.
B)no heat can flow between the system and its surroundings.
C)the speed of the wave is directly proportional to the bulk modulus.
D)the speed of the wave is proportional to the square root of the bulk modulus.
E)air is an ideal gas.
the time associated with heat conduction is slow relative to the speed of the wave.
4
The air in an automobile engine at 20 C is compressed from an initial pressure of 1.0 atm and a volume of 200 cm3 to a volume of 20 cm3. Find the temperature if the air behaves like an ideal gas ( = 1.4) and the compression is adiabatic.
A)730 C
B)460 C
C)25 C
D)50 C
E)20 C
A)730 C
B)460 C
C)25 C
D)50 C
E)20 C

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
5
The average molecular translational kinetic energy of a molecule in an ideal gas is:
A) kBT.
B) RT.
C) kBT.
D) RT.
E) kBT, where n = number of internal degrees of freedom.
A) kBT.
B) RT.
C) kBT.
D) RT.
E) kBT, where n = number of internal degrees of freedom.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
6
Nitrogen gas is heated by a pulsed laser to 50°000 K. If the diameter of the nitrogen atoms is assumed to be 1.0 * 10-10 m, and the pressure is 1.0 atm, what is the mean free path?
A)1.5 *10-4 m
B)1.5 * 10-7 m
C)1.5 * 10-10 m
D)1.5 * 10-14 m
E)1.5 * 10-2 m
A)1.5 *10-4 m
B)1.5 * 10-7 m
C)1.5 * 10-10 m
D)1.5 * 10-14 m
E)1.5 * 10-2 m

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
7
During an adiabatic compression, a volume of air decreases to 1/4 its original size. Calculate its final pressure if its original pressure was 1 atm. (Assume the air behaves like an ideal gas with = 1.4.)
A)7.0 atm
B)5.6 atm
C)3.5 atm
D)2.2 atm
E)0.14 atm
A)7.0 atm
B)5.6 atm
C)3.5 atm
D)2.2 atm
E)0.14 atm

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
8
The molar specific heat at constant volume at 0 C of an ideal monatomic gas is:
A) .
B)R.
C) .
D)2R.
E) .
A) .
B)R.
C) .
D)2R.
E) .

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
9
The molar specific heat at constant volume at 0 C of an ideal diatomic gas is:
A) .
B)R.
C) .
D)2R.
E) .
A) .
B)R.
C) .
D)2R.
E) .

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
10
Assume 3.0 moles of a diatomic gas has an internal kinetic energy of 10 kJ. Determine the temperature of the gas after it has reached equilibrium.
A)270 K
B)160 K
C)800 K
D)1550 K
E)400 K
A)270 K
B)160 K
C)800 K
D)1550 K
E)400 K

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
11
The average kinetic energy of a nitrogen molecule at room temperature (20 C) is:
A)2 *10-21 J.
B)4 * 10-21 J.
C)6 * 10-21 J.
D)8 * 10-21 J.
E)1 *10-20 J.
A)2 *10-21 J.
B)4 * 10-21 J.
C)6 * 10-21 J.
D)8 * 10-21 J.
E)1 *10-20 J.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
12
An ideal gas is allowed to expand adiabatically until its volume increases by 50%. By approximately what factor is the pressure reduced? ( = 5/3.)
A)1.5
B)2.0
C)2.5
D)3.0
E)3.5
A)1.5
B)2.0
C)2.5
D)3.0
E)3.5

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
13
A container having a volume of 1.0 m3 holds 5.0 moles of helium gas at 50 C. If the helium behaves like an ideal gas, the total energy of the system is:
A)2.0* 104 J.
B)2.5 *104 J.
C)1.7 * 103 J.
D)1.5 *103 J.
E)4.0 * 104 J.
A)2.0* 104 J.
B)2.5 *104 J.
C)1.7 * 103 J.
D)1.5 *103 J.
E)4.0 * 104 J.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
14
A container having a volume of 1.0 m3 holds 5.0 moles of helium gas at 50 C. If the helium behaves like an ideal gas, the average kinetic energy per molecule is:
A)6.7* 10-20 J.
B)1.0 * 10-21 J.
C)1.0 * 10-20 J.
D)6.7 * 10-21 J.
E)1.3* 10-20 J.
A)6.7* 10-20 J.
B)1.0 * 10-21 J.
C)1.0 * 10-20 J.
D)6.7 * 10-21 J.
E)1.3* 10-20 J.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
15
Find the specific heat (in J/mole K) of a gas kept at constant volume when it takes 1.0 *104 J of heat to raise the temperature of 5.0 moles of the gas 200 K above the initial temperature.
A)31
B)21
C)10
D)42
E)84
A)31
B)21
C)10
D)42
E)84

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
16
The theorem of equipartition of energy states that the energy each degree of freedom contributes to each molecule in the system (an ideal gas) is:
A) .
B) .
C) .
D) .
E) .
A) .
B) .
C) .
D) .
E) .

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
17
Which statement below is NOT an assumption made in the molecular model of an ideal gas?
A)The average separation between molecules is large compared with the dimensions of the molecules.
B)The molecules undergo inelastic collisions with one another.
C)The forces between molecules are short range.
D)The molecules obey Newton's laws of motion.
E)Any molecule can move in any direction with equal probability.
A)The average separation between molecules is large compared with the dimensions of the molecules.
B)The molecules undergo inelastic collisions with one another.
C)The forces between molecules are short range.
D)The molecules obey Newton's laws of motion.
E)Any molecule can move in any direction with equal probability.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
18
Assume molecules have an average diameter of 3.00 * 10-10 m. How many times larger is the mean free path than the diameter at one atmosphere and 0 C?
A)500
B)300
C)700
D)1000
E)2500
A)500
B)300
C)700
D)1000
E)2500

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
19
The internal energy of n moles of an ideal gas depends on:
A)one state variable T.
B)two state variables T and V.
C)two state variables T and P.
D)three state variables T, P and V.
E)four variables R, T, P and V.
A)one state variable T.
B)two state variables T and V.
C)two state variables T and P.
D)three state variables T, P and V.
E)four variables R, T, P and V.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
20
Suppose a box contains about 5.0* 1021 hydrogen atoms at room temperature (21 C). Determine the thermal energy of these atoms.
A)10 J
B)20 J
C)30 J
D)5.0 J
E)1.0 J
A)10 J
B)20 J
C)30 J
D)5.0 J
E)1.0 J

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
21
A 50-gram sample of dry ice (solid CO2) is placed in a 4-litre container. The system is sealed and allowed to reach room temperature (20 C). By approximately how much does the pressure inside the container increase when the dry ice turns to gas? (Ignore the initial volume of the sample.)

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
22
The root mean square speed of a gas molecule is greater than the average speed, because the former gives a greater weight to:
A)lighter molecules.
B)heavier molecules.
C)lower speeds.
D)higher speeds.
E)more probable speeds.
A)lighter molecules.
B)heavier molecules.
C)lower speeds.
D)higher speeds.
E)more probable speeds.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
23
Two tanks of gas, one of hydrogen, H2, and one of helium, He, contain equal numbers of moles of gas. The gram-molecular mass of He is twice that of H2. Both tanks of gas are at the same temperature, 293 K. Which statement(s) below is(are) correct when we ignore vibrational motion?
A)The total internal energy of the hydrogen is the same as that of the helium.
B)The total internal energy of the hydrogen is 1.4 times that of the helium.
C)The total internal energy of the helium is 1.4 times that of the hydrogen.
D)The total internal energy of the hydrogen is 1.67 times that of the helium.
E)The total internal energy of the helium is 1.67 times that of the hydrogen.
A)The total internal energy of the hydrogen is the same as that of the helium.
B)The total internal energy of the hydrogen is 1.4 times that of the helium.
C)The total internal energy of the helium is 1.4 times that of the hydrogen.
D)The total internal energy of the hydrogen is 1.67 times that of the helium.
E)The total internal energy of the helium is 1.67 times that of the hydrogen.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
24
Two tanks of gas, one of hydrogen, H2, and one of helium, He, contain equal masses of gas. The gram-molecular mass of He is twice that of H2. Both tanks of gas are at the same temperature, 293 K. Which statement(s) below is(are) correct when we ignore vibrational motion?
A)The total internal energy of the hydrogen is the same as that of the helium.
B)The total internal energy of the hydrogen is 167 times that of the helium.
C)The total internal energy of the helium is 1.67 times that of the hydrogen.
D)The total internal energy of the hydrogen is 3.33 times that of the helium.
E)The total internal energy of the helium is 3.33 times that of the hydrogen.
A)The total internal energy of the hydrogen is the same as that of the helium.
B)The total internal energy of the hydrogen is 167 times that of the helium.
C)The total internal energy of the helium is 1.67 times that of the hydrogen.
D)The total internal energy of the hydrogen is 3.33 times that of the helium.
E)The total internal energy of the helium is 3.33 times that of the hydrogen.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
25
If CP for an ideal gas is 35.4 J/mol.K, which of the following is CV for this gas?
A)12.5 J/mol.K
B)20.8 J/mol.K
C)29.1 J/mol.K
D)27.1 J/mol.K
E)43.4 J/mol.K
A)12.5 J/mol.K
B)20.8 J/mol.K
C)29.1 J/mol.K
D)27.1 J/mol.K
E)43.4 J/mol.K

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
26
The specific heat of an ideal gas at constant pressure is greater than the specific heat of an ideal gas at constant volume because:
A)work is done by a gas at constant pressure.
B)work is done by a gas at constant volume.
C)no work is done by a gas at constant pressure.
D)the temperature remains constant for a gas at constant pressure.
E)the temperature remains constant for a gas at constant volume.
A)work is done by a gas at constant pressure.
B)work is done by a gas at constant volume.
C)no work is done by a gas at constant pressure.
D)the temperature remains constant for a gas at constant pressure.
E)the temperature remains constant for a gas at constant volume.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
27
Air expands adiabatically (no heat in, no heat out) from T = 300 K and P = 100 atm to a final pressure of 1 atm. Treat the gas as ideal with = 1.4, and determine the final temperature. Compare your result to the boiling points of nitrogen (77.4 K) and oxygen (90.2 K). Could this method result in the liquification of air?

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
28
If the rms speed of helium atoms is vrms,He at temperature T, what is the rms speed of CO2 at the same temperature?
A)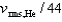
B)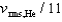
C)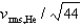
D)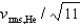
E)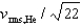
A)
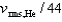
B)
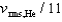
C)
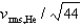
D)
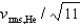
E)
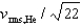

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
29
One mole of helium gas expands adiabatically from 2 atm pressure to 1 atm pressure. If the original temperature of the gas is 20 C, what is the final temperature of the gas? (F = 1.67)

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
30
The molar specific heat at constant pressure at 0 C of an ideal monatomic gas is:
A) .
B)R.
C) .
D)2R.
E) .
A) .
B)R.
C) .
D)2R.
E) .

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
31
If the total translational kinetic energy of the molecules of oxygen in a container is 15 J at room temperature, what is the total rotational kinetic energy of these molecules?
A)5 J
B)10 J
C)20 J
D)25 J
E)0 J
A)5 J
B)10 J
C)20 J
D)25 J
E)0 J

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
32
The temperature of a quantity of an ideal gas is:
A)one measure of its ability to transfer thermal energy to another body.
B)proportional to the average molecular kinetic energy of the molecules.
C)proportional to the internal energy of the gas.
D)correctly described by all the statements above.
E)correctly described only by (a) and (b) above.
A)one measure of its ability to transfer thermal energy to another body.
B)proportional to the average molecular kinetic energy of the molecules.
C)proportional to the internal energy of the gas.
D)correctly described by all the statements above.
E)correctly described only by (a) and (b) above.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
33
According to kinetic theory, a typical gas molecule in thermal equilibrium at room temperature has a kinetic energy K = 6.00 * 10-21 J, regardless of mass. Estimate the speed at room temperature of a hydrogen molecule H2 (m = 3.34 *10-27 kg) and a xenon atom (m = 2.00 *10-25 kg). [kB = 1.38 * 10-23 J/K]

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
34
During the volcanic eruption of Mt Pelee in 1902, an incredibly hot 'burning cloud' rolled down the mountain and incinerated the town of Saint-Pierre. From the damage done, the temperature in the cloud was estimated at 700 C. If the air temperature was 20 C and a mole of air is 29 grams, estimate the molecular weight of the gas in the 'burning cloud' that made it heavier than the surrounding air. (As a follow-on, estimate the most probable composition of the cloud. Some typical volcanic gases are H2S, SO2, H2SO4, CO2, NO.)

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck
35
When we consider a thin horizontal layer of the atmosphere, of thickness dy, of area A, with pressure P on the bottom, with an average mass m per molecule, and nV molecules per unit volume, the magnitude of the difference of the pressure at the top and bottom of the layer is given by dP =
A)mgdy.
B)mgnVdy.
C)mgAdy.
D)mgnVAdy.
E)mgnVAPdy.
A)mgdy.
B)mgnVdy.
C)mgAdy.
D)mgnVAdy.
E)mgnVAPdy.

Unlock Deck
Unlock for access to all 35 flashcards in this deck.
Unlock Deck
k this deck


