Deck 1: Energy Basics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 1: Energy Basics
1
Compare the energy scales of mechanical, chemical, and mass energy by
calculating (a) the energy associated with dropping 1 kg of coal through a vertical distance
of 100 m, (b) burning 1 kg of coal, and (c) converting the mass of 1 kg of coal into energy.
calculating (a) the energy associated with dropping 1 kg of coal through a vertical distance
of 100 m, (b) burning 1 kg of coal, and (c) converting the mass of 1 kg of coal into energy.
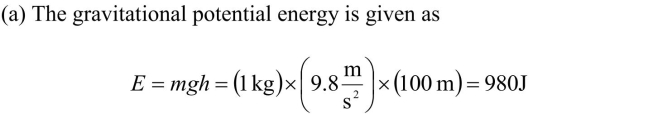
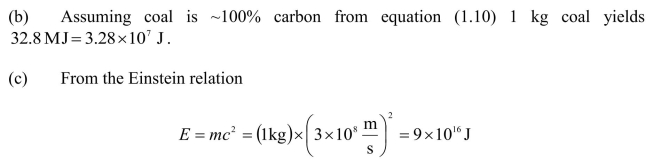 These values emphasize the relative magnitudes of mechanical, chemical and mass energy.
These values emphasize the relative magnitudes of mechanical, chemical and mass energy.While it may not be surprising that the mass energy is many orders of magnitude greater
than the other forms of energy, it is interesting to note that the chemical energy is more
than 30,000 times the gravitational energy in this example.
2
Calculate the ideal Carnot efficiency of a steam turbine that utilizes steam at 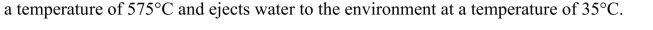
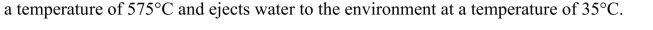
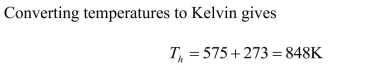 and
and 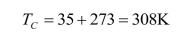

3
(a) Estimate the energy required to raise the temperature of a cup of coffee
from room temperature to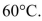 (b) Estimate the mass energy contained in a cup of hot
(b) Estimate the mass energy contained in a cup of hot
coffee.
from room temperature to
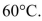 (b) Estimate the mass energy contained in a cup of hot
(b) Estimate the mass energy contained in a cup of hotcoffee.
(a) Assume a cup of hot coffee has a mass of 200 g and a room temperature of 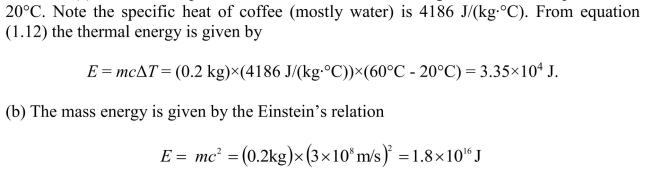
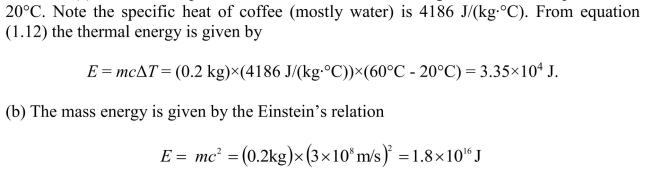
4
One homeowner uses electricity to heat a home (with 100% efficiency) at
an annual cost of $2600. A second homeowner uses a heat pump with a coefficient of
performance of 7 to heat an identical home. What is the second homeowner's annual
heating cost?
an annual cost of $2600. A second homeowner uses a heat pump with a coefficient of
performance of 7 to heat an identical home. What is the second homeowner's annual
heating cost?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
The lower heating value (LHV) is defined as the heat that is available from
the combustion of a fuel excluding the latent heat of vaporization of the steam produced.
From equation (1.16) determine the LHV of ethanol.
the combustion of a fuel excluding the latent heat of vaporization of the steam produced.
From equation (1.16) determine the LHV of ethanol.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
A coal-fired power plant operates at an efficiency of 38%. If the facility
produces 6 TWh of electricity per year, what is the rate of coal consumption in kg/s.
Approximate coal as pure carbon.
produces 6 TWh of electricity per year, what is the rate of coal consumption in kg/s.
Approximate coal as pure carbon.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
Water flows through a 1 m diameter pipe at a rate of 0.5 m per second. If the 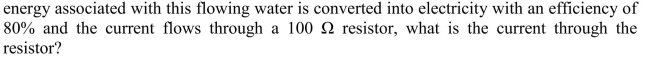
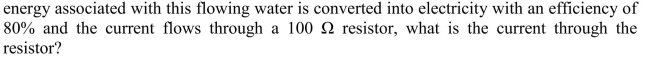

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
A 500 W electric heater is used to heat a 100 L tank of water with an
efficiency of 100% and no heat loss. How long will it take to raise the temperature of the
water by 20°C?
efficiency of 100% and no heat loss. How long will it take to raise the temperature of the
water by 20°C?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
A heat pump with a coefficient of performance of 8.5 is used to heat a 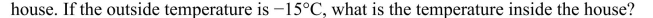
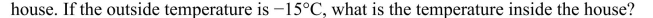

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
One gram of methane is burned, and the heat is used to raise the temperature
of 1 kg of water. If the initial temperature of the water is 25°C, what is the final
temperature?
of 1 kg of water. If the initial temperature of the water is 25°C, what is the final
temperature?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
A simple way of looking at the energy associated with the combustion of
methane [as shown in equation (1.15)] is to view the oxidation of the carbon by equation
(1.13) and the oxidation of hydrogen by equation (1.19). Based on the energies involved in
these processes, discuss the validity of this approach.
methane [as shown in equation (1.15)] is to view the oxidation of the carbon by equation
(1.13) and the oxidation of hydrogen by equation (1.19). Based on the energies involved in
these processes, discuss the validity of this approach.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
Steam at a temperature of 350°C is used to run a turbine. If the exhaust is at 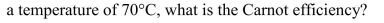
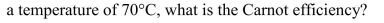

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
An average person can produce considerable power output for short periods 


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
A power transmission line is used to transmit 100 kW of power at a voltage 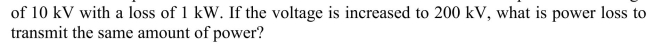
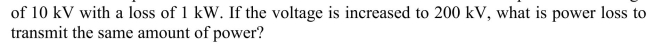

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
One cubic meter of water is poured off a 100-m high bridge. If the change in
gravitational potential energy is converted into electricity with an efficiency of 90%, how
long can this energy illuminate a standard 60-W light bulb?
gravitational potential energy is converted into electricity with an efficiency of 90%, how
long can this energy illuminate a standard 60-W light bulb?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
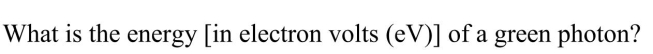

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
(a) If 15 kW of power from a heat reservoir at 500 K is input into a heat
engine with an efficiency of 37%, what is the power output?
(b) What is the temperature of the cold reservoir?
engine with an efficiency of 37%, what is the power output?
(b) What is the temperature of the cold reservoir?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
The temperature of 1 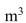 of water is decreased by
of water is decreased by 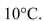 If this thermal
If this thermal
energy is used to lift the water vertically against gravity, what is the change in height of the
center of mass?
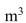 of water is decreased by
of water is decreased by 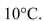 If this thermal
If this thermalenergy is used to lift the water vertically against gravity, what is the change in height of the
center of mass?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
A local FM radio station broadcasts at 104.3 MHz. What is the wavelength
of the radio waves?
of the radio waves?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
A rotating solid disk with a diameter of 0.25 m and a mass of 10 kg is used
to store 1 kJ of energy. What is its rotational speed in revolutions per minute?
to store 1 kJ of energy. What is its rotational speed in revolutions per minute?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck


