Deck 3: Fossil Fuel Resources and Use
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 3: Fossil Fuel Resources and Use
1
A large passenger ship weighs 75,000 tonnes and has a cruising speed of 45
km/h. The engines burn diesel fuel (approximately the same energy content as crude oil)
with an efficiency of 20%. The drive system has an average efficiency of 55% for
converting mechanical energy into propulsion for the ship. Calculate the volume of fuel
needed to accelerate the ship from rest to cruising speed.
km/h. The engines burn diesel fuel (approximately the same energy content as crude oil)
with an efficiency of 20%. The drive system has an average efficiency of 55% for
converting mechanical energy into propulsion for the ship. Calculate the volume of fuel
needed to accelerate the ship from rest to cruising speed.
At rest the kinetic energy is zero. At 45 km/h 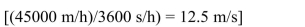 the kinetic energy will be
the kinetic energy will be 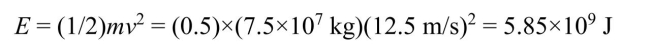

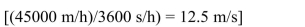 the kinetic energy will be
the kinetic energy will be 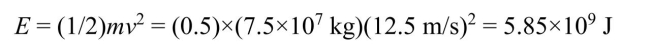

2
A person uses the energy equivalent of 50 bbl of crude oil in one year. What
is that person's average power consumption?
is that person's average power consumption?
1 bbl of crude oil has the energy content 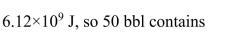
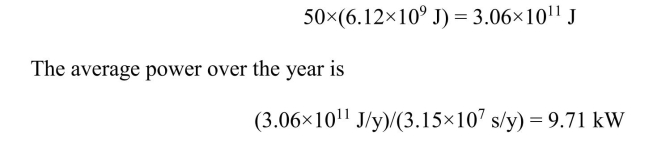
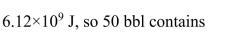
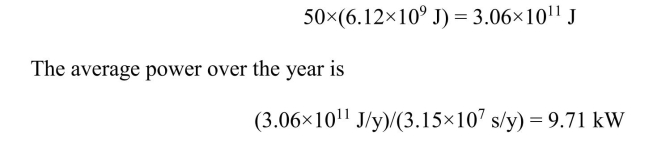
3
A coal-fired generating station has an efficiency of 38% and produces an
average electrical output of 1000 MWe.
(a) How much coal does it burn per second? Assume the coal is bituminous.(b) How much
heat (in joules) is released to the environment per second?
(c) How many liters of water at 20°C could the heat in part (b) boil (at STP) per second?
average electrical output of 1000 MWe.
(a) How much coal does it burn per second? Assume the coal is bituminous.(b) How much
heat (in joules) is released to the environment per second?
(c) How many liters of water at 20°C could the heat in part (b) boil (at STP) per second?
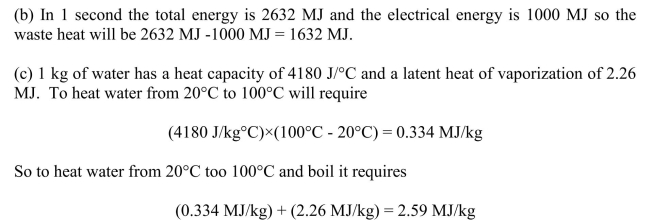
(a) 1000 MWe output at 38% efficiency requires 1000 MW)/(0.38) = 2632 MW
thermal energy from the combustion of coal. Using an energy content of coal of 31 MJ/kg
then the mass of coal required per second will be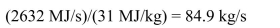
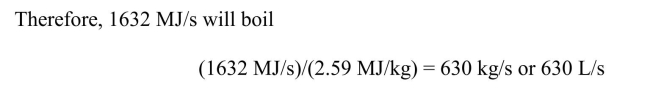
thermal energy from the combustion of coal. Using an energy content of coal of 31 MJ/kg
then the mass of coal required per second will be
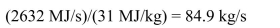

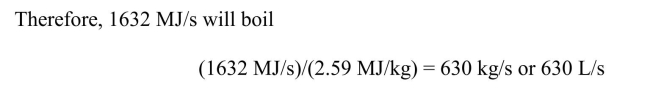
4
Write down the chemical formulae for the combustion of the alkanes in Table 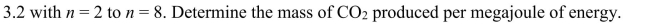

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
A portion of the Keystone Pipeline connecting oil production in Canada and
refining in the United States consists of a 0.76 m diameter pipe that carries 500,000 bbl of
crude oil per day over a distance of 1744 km.
(a) Calculate the average velocity of the oil in the pipe.
(b) What is the rate of chemical energy flow through the pipe (in watts) based on the
velocity of the oil and its energy content?
refining in the United States consists of a 0.76 m diameter pipe that carries 500,000 bbl of
crude oil per day over a distance of 1744 km.
(a) Calculate the average velocity of the oil in the pipe.
(b) What is the rate of chemical energy flow through the pipe (in watts) based on the
velocity of the oil and its energy content?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
On land, coal is transported primarily by train. A typical large coal train may
be about 1.5 km long and may consist of 120 cars, each holding 110 tonnes of coal. As
each tonne of coal has an equivalent energy content (in terms of the stored chemical energy
it contains), a moving coal train represents a flow of (chemical) energy or a power. For a
coal train traveling at 100 km/h, calculate the equivalent power in watts.
be about 1.5 km long and may consist of 120 cars, each holding 110 tonnes of coal. As
each tonne of coal has an equivalent energy content (in terms of the stored chemical energy
it contains), a moving coal train represents a flow of (chemical) energy or a power. For a
coal train traveling at 100 km/h, calculate the equivalent power in watts.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
(a) Locate, from an appropriate source, the composition for methanol and
ethanol. Write down the formulas for the combustion of these two hydrocarbons.
(b) Calculate the mass of CO2 produced per kg of methanol and ethanol burned.
(c) Locate values for the heat of combustion of methanol and ethanol and determine the
mass of CO2 produced per MJ of energy.
ethanol. Write down the formulas for the combustion of these two hydrocarbons.
(b) Calculate the mass of CO2 produced per kg of methanol and ethanol burned.
(c) Locate values for the heat of combustion of methanol and ethanol and determine the
mass of CO2 produced per MJ of energy.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
Assume that the total energy needs of a person in the United States (Chapter
2) is satisfied by burning coal. If each person is responsible for the mining, transportation,
processing, and burning of their own coal, how much coal must each person process, on
average, per day?
2) is satisfied by burning coal. If each person is responsible for the mining, transportation,
processing, and burning of their own coal, how much coal must each person process, on
average, per day?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
Locate the current world price of oil (US$/bbl), coal (bituminous coal, per
tonne), and natural gas (per 1000 m3). For each fossil fuel, calculate the cost of energy per
gigajoule assuming that the fuel can be converted to usable energy with an efficiency of
100%.
tonne), and natural gas (per 1000 m3). For each fossil fuel, calculate the cost of energy per
gigajoule assuming that the fuel can be converted to usable energy with an efficiency of
100%.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
Estimate the total coal produced in the United Kingdom between 1820 and
the present.
the present.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
Liquid natural gas is transported by ship. A typical LNG tanker may have a 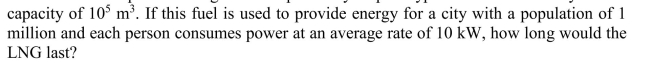
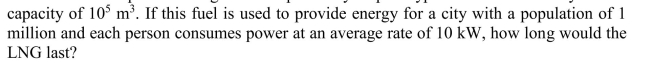

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
Locate the current world price of oil (US$/bbl) and the current retail price of
regular gasoline in your area (per liter or per gallon, as appropriate). What is the markup in
the price per unit energy between crude oil and retail gasoline?
regular gasoline in your area (per liter or per gallon, as appropriate). What is the markup in
the price per unit energy between crude oil and retail gasoline?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
Using the information in Figure 2.6 and the average composition of coal
(lignite, sub-bituminous/bituminous and anthracite) in the United States, calculate the mass
of the coal used annually. Use the average energy content for sub-bituminous/bituminous
coal.
(lignite, sub-bituminous/bituminous and anthracite) in the United States, calculate the mass
of the coal used annually. Use the average energy content for sub-bituminous/bituminous
coal.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
How many tonnes of oil shale which produces 120 L/t would be needed to
produce the same energy as 1 tonne of bituminous coal?
produce the same energy as 1 tonne of bituminous coal?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
Find the retail cost of gasoline (per unit volume) in your area and find the
cost of electricity for residential users in your area.
(a) Compare the cost of energy (per MJ) from gasoline and from electricity from the public
utility.
(b) The efficiency of a gasoline automobile is about 20% and the efficiency of a battery
electric vehicle is about 85%. Discuss the relative transportation costs for gasoline and
electric vehicles.
cost of electricity for residential users in your area.
(a) Compare the cost of energy (per MJ) from gasoline and from electricity from the public
utility.
(b) The efficiency of a gasoline automobile is about 20% and the efficiency of a battery
electric vehicle is about 85%. Discuss the relative transportation costs for gasoline and
electric vehicles.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
A coal-fired generating station consumes 2 × 106 tonnes of coal per year and
produces an average electrical output of 800 MWe. Calculate the plant's efficiency.
produces an average electrical output of 800 MWe. Calculate the plant's efficiency.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
For the reaction shown in equation (3.4), calculate the mass of CO and the
mass of H2 produced by the gasification of 1 kg of carbon. What is the energy content of
these two products?
mass of H2 produced by the gasification of 1 kg of carbon. What is the energy content of
these two products?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
If shale oil replaced coal in the United States, how many years would the
U.S. resources last at the current rate of use?
U.S. resources last at the current rate of use?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
The combustion of pure hydrogen by the reaction 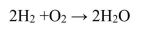
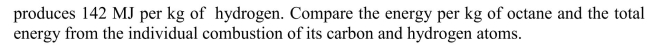

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
Assume that all of the United States' annual electricity requirement of 3×1012
kWh is produced by coal-fired generating stations operating at a net overall efficiency of
40%.
(a) How many tonnes of coal are burned per second? (Assume the coal is all bituminous)
(b) Assuming that coal is 100% carbon, how many tonnes of CO2 will be produced each
year?
kWh is produced by coal-fired generating stations operating at a net overall efficiency of
40%.
(a) How many tonnes of coal are burned per second? (Assume the coal is all bituminous)
(b) Assuming that coal is 100% carbon, how many tonnes of CO2 will be produced each
year?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck


