Deck 5: Sun Light and Sun Atoms
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/135
Play
Full screen (f)
Deck 5: Sun Light and Sun Atoms
1
The neutral hydrogen atom consists of
A) one proton and one neutron.
B) one proton.
C) one proton, one neutron, and one electron.
D) one proton and one electron.
E) an isotope and an ion.
A) one proton and one neutron.
B) one proton.
C) one proton, one neutron, and one electron.
D) one proton and one electron.
E) an isotope and an ion.
one proton and one electron.
2
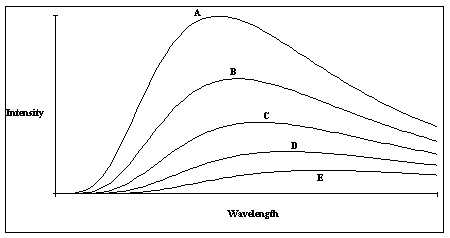 A plot of the continuous spectra of five different stars is shown in the figure. Based on these spectra, which of the stars is the hottest?
A plot of the continuous spectra of five different stars is shown in the figure. Based on these spectra, which of the stars is the hottest?A) Star A
B) Star B
C) Star C
D) Star D
E) Star E
Star A
3
The ____ of a gas is a measure of the average speed of the particles (atoms or molecules) in the gas.
A) heat
B) composition
C) temperature
D) blue shift
E) binding energy
A) heat
B) composition
C) temperature
D) blue shift
E) binding energy
temperature
4
Why don t we see hydrogen Balmer lines in the spectra of stars with temperatures of 45,000 K?
A) There is no hydrogen in stars this hot.
B) The stars are hot enough that most of the hydrogen is ionized and the atoms can not absorb energy.
C) These stars are so cool that nearly all of the electrons in the hydrogen atom are in the ground state.
D) Stars of this temperature are too cool to produce an absorption spectrum.
E) Stars of this temperature are too hot to produce an absorption spectrum.
A) There is no hydrogen in stars this hot.
B) The stars are hot enough that most of the hydrogen is ionized and the atoms can not absorb energy.
C) These stars are so cool that nearly all of the electrons in the hydrogen atom are in the ground state.
D) Stars of this temperature are too cool to produce an absorption spectrum.
E) Stars of this temperature are too hot to produce an absorption spectrum.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
5
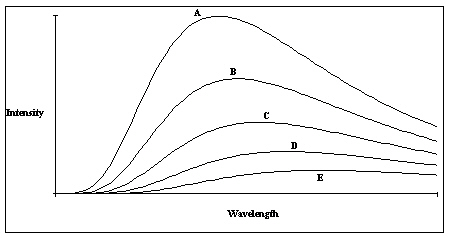 A plot of the continuous spectra of five different stars is shown in the figure. Based on these spectra, which of the stars has the lowest temperature?
A plot of the continuous spectra of five different stars is shown in the figure. Based on these spectra, which of the stars has the lowest temperature?A) Star A
B) Star B
C) Star C
D) Star D
E) Star E

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
6
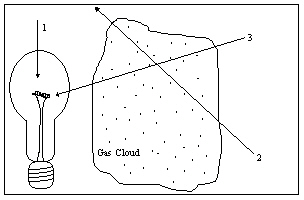 The diagram illustrates a light source, a gas cloud, and three different lines of sight. Along which line of sight would an observer see an emission spectrum?
The diagram illustrates a light source, a gas cloud, and three different lines of sight. Along which line of sight would an observer see an emission spectrum?A) 1
B) 2
C) 3
D) 2 and 3
E) none of them

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
7
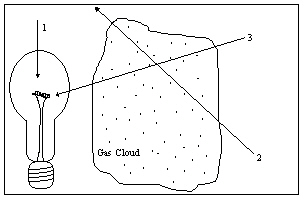 The diagram illustrates a light source, a gas cloud, and three different lines of sight. Along which line of sight would an observer see a continuous spectrum?
The diagram illustrates a light source, a gas cloud, and three different lines of sight. Along which line of sight would an observer see a continuous spectrum?A) 1
B) 2
C) 3
D) 2 and 3
E) none of them

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
8
Absolute zero is
A) zero degrees Celsius.
B) the temperature at which atoms have no remaining energy from which we can extract heat.
C) the temperature at which water freezes.
D) both a and c
E) none of the above
A) zero degrees Celsius.
B) the temperature at which atoms have no remaining energy from which we can extract heat.
C) the temperature at which water freezes.
D) both a and c
E) none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
9
The lowest energy level in an atom is
A) the absolute zero temperature.
B) the ground state.
C) the ionization level.
D) responsible for Doppler shifts.
E) the energy level from which the Paschen series of hydrogen originates.
A) the absolute zero temperature.
B) the ground state.
C) the ionization level.
D) responsible for Doppler shifts.
E) the energy level from which the Paschen series of hydrogen originates.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following can be determined by using the Doppler Effect?
I. The speed at which a star is moving away from an observer.
II. The transverse velocity of a star.
III. The radial velocity of a star.
IV. The speed at which a car is traveling toward an observer.
A) I, IV
B) II III
C) II IV
D) I,III
E) I, III, IV
I. The speed at which a star is moving away from an observer.
II. The transverse velocity of a star.
III. The radial velocity of a star.
IV. The speed at which a car is traveling toward an observer.
A) I, IV
B) II III
C) II IV
D) I,III
E) I, III, IV

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
11
The process of removing an electron from a stable nucleus is known as
A) ionization.
B) Doppler broadening.
C) collisional broadening.
D) a red shift.
E) quantum mechanics.
A) ionization.
B) Doppler broadening.
C) collisional broadening.
D) a red shift.
E) quantum mechanics.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
12
An atom that is excited
A) is also ionized.
B) is an isotope.
C) has had its electron moved to the lowest energy level.
D) can emit a photon when the electron moves to a lower energy level.
E) can emit a photon when the electron moves to a higher energy level.
A) is also ionized.
B) is an isotope.
C) has had its electron moved to the lowest energy level.
D) can emit a photon when the electron moves to a lower energy level.
E) can emit a photon when the electron moves to a higher energy level.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
13
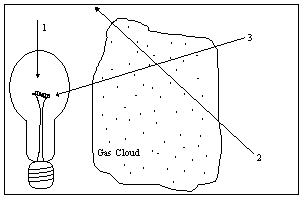 The diagram illustrates a light source, a gas cloud, and three different lines of sight. Along which line of sight would an observer see an absorption spectrum?
The diagram illustrates a light source, a gas cloud, and three different lines of sight. Along which line of sight would an observer see an absorption spectrum?A) 1
B) 2
C) 3
D) 2 and 3
E) none of them

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
14
Why don t we see hydrogen Balmer lines in the spectra of stars with temperatures of 3200 K?
A) There is no hydrogen in stars this cool.
B) The stars are hot enough that most of the hydrogen is ionized and the atoms cannot absorb energy.
C) These stars are so cool that nearly all of the electrons in the hydrogen atom are in the ground state.
D) Stars of this temperature are too cool to produce an absorption spectrum.
E) Stars of this temperature are too hot to produce an absorption spectrum.
A) There is no hydrogen in stars this cool.
B) The stars are hot enough that most of the hydrogen is ionized and the atoms cannot absorb energy.
C) These stars are so cool that nearly all of the electrons in the hydrogen atom are in the ground state.
D) Stars of this temperature are too cool to produce an absorption spectrum.
E) Stars of this temperature are too hot to produce an absorption spectrum.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
15
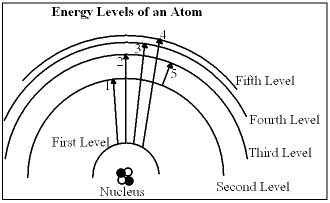 In the diagram, which of the transitions would absorb a photon with the smallest energy (longest wavelength)?
In the diagram, which of the transitions would absorb a photon with the smallest energy (longest wavelength)?A) Transition 1
B) TransitioN2
C) Transition 3
D) Transition 4
E) Transition 5

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
16
An atom can be excited
A) if it emits a photon.
B) if it collides with another atom or electron.
C) if it absorbs a photon.
D) a and b above
E) b and c above
A) if it emits a photon.
B) if it collides with another atom or electron.
C) if it absorbs a photon.
D) a and b above
E) b and c above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
17
The two most abundant elements in the sun are
A) nitrogen and oxygen.
B) hydrogen and helium.
C) sulfur and iron.
D) carbon and hydrogen.
E) carbon and nitrogen.
A) nitrogen and oxygen.
B) hydrogen and helium.
C) sulfur and iron.
D) carbon and hydrogen.
E) carbon and nitrogen.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
18
A(n) ____ contains two or more atoms that are bound together by exchanging or sharing electrons with each other.
A) nucleus
B) ion
C) proton
D) electron cloud
E) molecule
A) nucleus
B) ion
C) proton
D) electron cloud
E) molecule

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
19
You are standing near a railroad track and a train is moving toward you at 60 mph and blowing its horn. What will you notice as the train moves past you?
A) As the train approaches, the horn will sound lower in pitch than when the train is moving away.
B) As the train approaches, the horn will sound higher in pitch than when the train is moving away.
C) There will be no change in the pitch of the horn as it moves by.
D) The horn will get louder as the train moves away from you.
E) The horn will get quieter as the train moves toward you.
A) As the train approaches, the horn will sound lower in pitch than when the train is moving away.
B) As the train approaches, the horn will sound higher in pitch than when the train is moving away.
C) There will be no change in the pitch of the horn as it moves by.
D) The horn will get louder as the train moves away from you.
E) The horn will get quieter as the train moves toward you.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
20
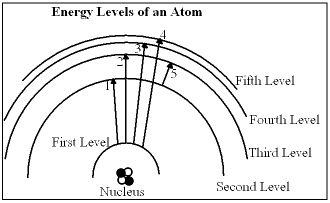 In the diagram, which of the transitions would absorb a photon with the greatest energy (shortest wavelength)?
In the diagram, which of the transitions would absorb a photon with the greatest energy (shortest wavelength)?A) Transition 1
B) TransitioN2
C) Transition 3
D) Transition 4
E) Transition 5

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
21
The H g line has a wavelength of 434.0 nm when observed in the laboratory. If the H g line appears in a stars spectrum at 434.5 nm, what is the radial velocity of the star?
A) 346 km/sec away from the observer.
B) 346 km/sec toward the observer.
C) 1.3*108m/sec away from the observer.
D) 1.3*108 m/sec toward the observer.
E) The radial velocity of the star can not be determined from this information.
A) 346 km/sec away from the observer.
B) 346 km/sec toward the observer.
C) 1.3*108m/sec away from the observer.
D) 1.3*108 m/sec toward the observer.
E) The radial velocity of the star can not be determined from this information.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
22
The sun has a surface temperature of approximately 5800 K. At what wavelength does the maximum energy radiated by the sun occur?
A) 5800 nm
B) 300 nm
C) 174 nm
D) 520 nm
E) 3000 nm
A) 5800 nm
B) 300 nm
C) 174 nm
D) 520 nm
E) 3000 nm

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
23
A neutral atom always contains
A) the same number of protons as it does neutrons.
B) the same number of electrons as it does neutrons.
C) the same number of protons as it does electrons.
D) twice as many protons as it does neutrons.
E) twice as many neutrons as it does protons.
A) the same number of protons as it does neutrons.
B) the same number of electrons as it does neutrons.
C) the same number of protons as it does electrons.
D) twice as many protons as it does neutrons.
E) twice as many neutrons as it does protons.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
24
The ____ is responsible for binding the electrons to the nucleus.
A) Kirchhoff s law
B) ground state
C) temperature
D) Coulomb force
E) Balmer series
A) Kirchhoff s law
B) ground state
C) temperature
D) Coulomb force
E) Balmer series

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
25
____ has a negative charge and a mass about 1800 times small than a proton.
A) A neutron
B) An electron
C) A molecule
D) A nucleus
E) An isotope
A) A neutron
B) An electron
C) A molecule
D) A nucleus
E) An isotope

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
26
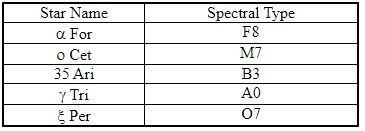
The table lists the spectral types for each of five stars. Which star in this table would have the greatest surface temperature?
A) a For
B) o Cet
C) 35 Ari
D) g Tri
E) x Per

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
27
One star has a temperature of 10,000 K and another star has a temperature of 5,000 K. Compared to the cooler star, how much more energy per second will the hotter star radiate from each square meter of its surface?
A) 16 times
B) 2 times
C) 1*1016times
D) 625 times
E) 25 times
A) 16 times
B) 2 times
C) 1*1016times
D) 625 times
E) 25 times

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
28
One star has a temperature of 30,000 K and another star has a temperature of 6,000 K. Compared to the cooler star, how much more energy per second will the hotter star radiate from each square meter of its surface?
A) 5 times
B) 25 times
C) 8.1*1017 times
D) 625 times
E) 1.*10 15 times
A) 5 times
B) 25 times
C) 8.1*1017 times
D) 625 times
E) 1.*10 15 times

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
29
At what wavelength would a star radiate the greatest amount of energy if the star has a surface temperature of 60,000 K?
A) 50 nm
B) 500 nm
C) 300 nm
D) 1.8*10 11 nm
E) 180 nm
A) 50 nm
B) 500 nm
C) 300 nm
D) 1.8*10 11 nm
E) 180 nm

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
30

The table lists the spectral types for each of five stars. Which star in this table would have the lowest surface temperature?
A) a For
B) o Cet
C) 35 Ari
D) g Tri
E) x Per

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
31
How much energy is radiated each second by one square meter of a star whose temperature is 10,000 K? s in the Stefan-Boltzmann law is equal to 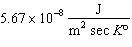 ,
,
A) 5.67*1012J
B) 5.67*108 J
C) 5.67*104 J
D) 300 nm
E) 300,000,000 nm
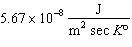 ,
,A) 5.67*1012J
B) 5.67*108 J
C) 5.67*104 J
D) 300 nm
E) 300,000,000 nm

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
32
The radiation emitted from a star has a maximum intensity at a wavelength of 300 nm. What is the temperature of this star?
A) 300 K
B) 100 K
C) 900,000,000 K
D) 90,000 K
E) 10,000 K
A) 300 K
B) 100 K
C) 900,000,000 K
D) 90,000 K
E) 10,000 K

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following cannot be determined from the spectrum of a star?
A) chemical composition
B) surface temperature
C) radial (along line of sight) velocity
D) tangential (perpendicular to line of sight) velocity
E) Both c and d
A) chemical composition
B) surface temperature
C) radial (along line of sight) velocity
D) tangential (perpendicular to line of sight) velocity
E) Both c and d

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
34
The H d line has a wavelength of 410.2 nm when observed in the laboratory. If the H d line appears in a stars spectrum at 410.0 nm, what is the radial velocity of the star?
A) 146 km/sec away from the observer.
B) 146 km/sec toward the observer.
C) 6.0*107m/sec away from the observer.
D) 6.0*107m/sec toward the observer.
E) The radial velocity of the star cannot be determined from this information.
A) 146 km/sec away from the observer.
B) 146 km/sec toward the observer.
C) 6.0*107m/sec away from the observer.
D) 6.0*107m/sec toward the observer.
E) The radial velocity of the star cannot be determined from this information.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
35
____ is a set of rules that describes how atoms and subatomic particles behave.
A) Kirchhoff s law
B) Black body radiation law
C) The Coulomb force
D) Quantum mechanics
E) The binding energy
A) Kirchhoff s law
B) Black body radiation law
C) The Coulomb force
D) Quantum mechanics
E) The binding energy

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
36
The temperature of an object from which no heat energy can be extracted is
A) 0 F
B) 0 C
C) 0 K
D) 100 K
E) 100 C
A) 0 F
B) 0 C
C) 0 K
D) 100 K
E) 100 C

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
37
The binding energy of the first level in an atom is 2.2 10 -18 J, and the binding energy of the second energy level is 1.6 10 -18 J. What is the energy of the photon that is emitted if an electron moves from the second level to the first?
A) 3.3*10-18 J
B) 3.5*10-36 J
C) 1.4 J
D) 3.5*10-18 J
E) 6.0*10-19 J
A) 3.3*10-18 J
B) 3.5*10-36 J
C) 1.4 J
D) 3.5*10-18 J
E) 6.0*10-19 J

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
38
Atoms that have the same number of protons but a different number of neutrons are called
A) ions.
B) molecules
C) atomic pairs.
D) nuclear pairs.
E) isotopes.
A) ions.
B) molecules
C) atomic pairs.
D) nuclear pairs.
E) isotopes.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
39
If you move an electron in an atom from a low energy level to a higher energy level within the atom, we say that the atom is
A) in the ground state.
B) ionized.
C) dissociated.
D) an excited atom.
E) neutralized.
A) in the ground state.
B) ionized.
C) dissociated.
D) an excited atom.
E) neutralized.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
40
The absorption lines in the visible portion of the spectrum of a star that are produced by hydrogen are from the
A) Lyman series.
B) Balmer series.
C) Paschen series.
D) isotopes of hydrogen.
E) ions of hydrogen.
A) Lyman series.
B) Balmer series.
C) Paschen series.
D) isotopes of hydrogen.
E) ions of hydrogen.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
41
The sun s magnetic field is evident in the looped shapes of
A) solar flares.
B) sunspots.
C) the corona.
D) granules.
E) prominences.
A) solar flares.
B) sunspots.
C) the corona.
D) granules.
E) prominences.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
42
Sunspots are known to be magnetic phenomena because
A) Doppler shifts in spectral lines are observed.
B) the Zeeman effect is observed in sunspots.
C) collisional broadening is observed in spectral lines.
D) infrared observations indicate that the sunspots are cooler than their surroundings.
E) observations during eclipses reveal a very extensive photosphere.
A) Doppler shifts in spectral lines are observed.
B) the Zeeman effect is observed in sunspots.
C) collisional broadening is observed in spectral lines.
D) infrared observations indicate that the sunspots are cooler than their surroundings.
E) observations during eclipses reveal a very extensive photosphere.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
43
Much of the solar wind comes from ____ where the magnetic field does not loop back into the sun.
A) prominences
B) coronal holes
C) spicules
D) granulation
E) auroras
A) prominences
B) coronal holes
C) spicules
D) granulation
E) auroras

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
44
The Doppler effect states that the motion of any object can
A) shift the wavelength of spectral lines.
B) change the speed of light emitted from the object.
C) enhance the chemical composition of the object.
D) make the object appear hotter.
E) make the object appear cooler.
A) shift the wavelength of spectral lines.
B) change the speed of light emitted from the object.
C) enhance the chemical composition of the object.
D) make the object appear hotter.
E) make the object appear cooler.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
45
Differential rotation of the sun is
A) heating in the chromosphere and corona that makes them hotter than the photosphere.
B) the magnetic dynamo inside the sun.
C) the equatorial regions of the sun rotating more rapidly than the polar regions.
D) the origin (and subsequent disappearance of) sunspots first near the poles then closer to the sun s equator as the sunspot cycle progresses.
E) the rotation of the sun s southern and northern hemispheres in opposite directions.
A) heating in the chromosphere and corona that makes them hotter than the photosphere.
B) the magnetic dynamo inside the sun.
C) the equatorial regions of the sun rotating more rapidly than the polar regions.
D) the origin (and subsequent disappearance of) sunspots first near the poles then closer to the sun s equator as the sunspot cycle progresses.
E) the rotation of the sun s southern and northern hemispheres in opposite directions.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
46
The corona and chromosphere of the sun are believed to be heated by
A) shock waves rising from below the photosphere.
B) the solar wind.
C) sunspots.
D) high energy particles being accelerated by the sun s magnetic field.
E) differential rotation.
A) shock waves rising from below the photosphere.
B) the solar wind.
C) sunspots.
D) high energy particles being accelerated by the sun s magnetic field.
E) differential rotation.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
47
The energy of a photon is proportional to the light s
A) wavelength.
B) speed.
C) frequency.
D) intensity.
E) two of the above.
A) wavelength.
B) speed.
C) frequency.
D) intensity.
E) two of the above.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
48
Granulation is caused by
A) sunspots.
B) rising and sink gases below the photosphere.
C) shock waves in the corona.
D) the solar wind flowing away from the corona.
E) the heating in the chromosphere.
A) sunspots.
B) rising and sink gases below the photosphere.
C) shock waves in the corona.
D) the solar wind flowing away from the corona.
E) the heating in the chromosphere.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
49
The chromosphere of the sun
A) is hotter than the photosphere.
B) appears yellow-white in color during total solar eclipse.
C) is the visible surface of the sun.
D) produces an absorption spectrum.
E) all of the above
A) is hotter than the photosphere.
B) appears yellow-white in color during total solar eclipse.
C) is the visible surface of the sun.
D) produces an absorption spectrum.
E) all of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
50
____ about 130 km above Earth s surface near the polar regions when energy in the solar wind guided by Earth s magnetic field excites gases in the upper atmosphere.
A) Coronas occur
B) Flares occur
C) Auroras occur
D) Coronal holes occur
E) Nuclear fission occurs
A) Coronas occur
B) Flares occur
C) Auroras occur
D) Coronal holes occur
E) Nuclear fission occurs

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
51
The bluer the light, the ____ each photon contains.
A) more energy
B) less energy
C) less speed
D) more speed
E) none of the above
A) more energy
B) less energy
C) less speed
D) more speed
E) none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
52
The sunspot cycle affects ____________.
I. the latitude at which most sunspots occur
II. the number of sunspots that are visible.
III. the rotation rate of the sun s equator
IV. the magnetic polarity of the sunspot pair members in a hemisphere.
A) I,II
B) I,IV
C) II,III
D) I, II III
E) I, II, IV
I. the latitude at which most sunspots occur
II. the number of sunspots that are visible.
III. the rotation rate of the sun s equator
IV. the magnetic polarity of the sunspot pair members in a hemisphere.
A) I,II
B) I,IV
C) II,III
D) I, II III
E) I, II, IV

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
53
The ____ coincides with the period known as the little ice age of Europe and North America. This provides one piece of evidence that suggests a link between solar activity and the amount of solar energy Earth receives.
A) Maunder sunspot minimum
B) Babcock sunspot model
C) coronal hole
D) Coulomb barrier
E) weak solar force
A) Maunder sunspot minimum
B) Babcock sunspot model
C) coronal hole
D) Coulomb barrier
E) weak solar force

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
54
Most of the visible light we see coming from the sun originates from the
A) chromosphere.
B) photosphere.
C) corona.
D) sunspots.
E) magnetic field.
A) chromosphere.
B) photosphere.
C) corona.
D) sunspots.
E) magnetic field.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
55
The strong force is the force that
A) binds electrons to the nucleus in an atom.
B) holds the moon in orbit around Earth.
C) creates the magnetic field associated with sunspots.
D) produces the extremely high temperatures in the solar corona.
E) binds protons and neutrons together to form a nucleus.
A) binds electrons to the nucleus in an atom.
B) holds the moon in orbit around Earth.
C) creates the magnetic field associated with sunspots.
D) produces the extremely high temperatures in the solar corona.
E) binds protons and neutrons together to form a nucleus.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
56
Astronomers can use ____ to measure magnetic fields on the sun.
A) helioseismology
B) perchloroethylene (C2Cl4)
C) neutrino detectors
D) a magnetic carpet
E) the Zeeman effect
A) helioseismology
B) perchloroethylene (C2Cl4)
C) neutrino detectors
D) a magnetic carpet
E) the Zeeman effect

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
57
A filtergram is a photograph of the sun s surface made
A) in a band of wavelengths in the infrared.
B) in a band of wavelengths in the ultraviolet.
C) using the Zeeman effect.
D) with only those photons emitted in a specific spectral line.
E) none of the above
A) in a band of wavelengths in the infrared.
B) in a band of wavelengths in the ultraviolet.
C) using the Zeeman effect.
D) with only those photons emitted in a specific spectral line.
E) none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
58
What is the order of star colors with increasing temperature?
A) Red, Yellow, Blue
B) Blue, Red, Yellow
C) Red, Blue, Yellow
D) Yellow, Red, Blue
E) Blue, Yellow, Red
A) Red, Yellow, Blue
B) Blue, Red, Yellow
C) Red, Blue, Yellow
D) Yellow, Red, Blue
E) Blue, Yellow, Red

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
59
The ____ occurs when a rapidly rotating conductor is stirred by convection to produce a magnetic field.
A) dynamo effect
B) Zeeman effect
C) Babcock effect
D) proton-proton chain
E) aurora
A) dynamo effect
B) Zeeman effect
C) Babcock effect
D) proton-proton chain
E) aurora

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
60
Each element has its own set of characteristic absorption lines because
A) the temperature of each element can varies.
B) elements can exist in different forms of matter.
C) electron energy levels differ for each element.
D) each element has a different mass.
E) absorption lines depend upon the speed of the object.
A) the temperature of each element can varies.
B) elements can exist in different forms of matter.
C) electron energy levels differ for each element.
D) each element has a different mass.
E) absorption lines depend upon the speed of the object.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
61
The United States consumes 2.5*1019 J of energy each year. A typical solar flare releases 5.0 10 24 J of energy. How many years could we run the United States on the energy released by this solar flare if all of the released energy could be used?
A) 5*10-6 years
B) 200,000 years
C) 1.2*1044 years
D) about 12 years
E) 500 years
A) 5*10-6 years
B) 200,000 years
C) 1.2*1044 years
D) about 12 years
E) 500 years

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
62
Sunspots
A) are cooler than their surroundings.
B) are regions where material is rising from below the photosphere.
C) are the result of convection.
D) produce spicules.
E) are generally found near the poles of the sun during sunspot maximum.
A) are cooler than their surroundings.
B) are regions where material is rising from below the photosphere.
C) are the result of convection.
D) produce spicules.
E) are generally found near the poles of the sun during sunspot maximum.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
63
The ____ is (are) the hot gases that are the moving extension of the sun s corona.
A) spicules
B) prominences
C) flares
D) supergranules
E) solar wind
A) spicules
B) prominences
C) flares
D) supergranules
E) solar wind

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
64
If a sunspot has a temperature of 4,500 K and the surrounding solar surface has a temperature of 5,800 K, how many times brighter is the surface compared to the sunspot?
A) 0.28
B) 0.36
C) 2.8
D) 3.6
E) 36
A) 0.28
B) 0.36
C) 2.8
D) 3.6
E) 36

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
65
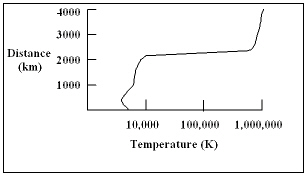 The diagram shows a plot of the temperature of the sun as a function of distance above the bottom of the photosphere. What is the temperature of the sun at a distance of 2,000 km?
The diagram shows a plot of the temperature of the sun as a function of distance above the bottom of the photosphere. What is the temperature of the sun at a distance of 2,000 km?A) 500 K
B) 900 K
C) 5,000 K
D) 9,000 K
E) 100,000 K

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
66
____ is (are) produced by atomic transitions in the presence of a strong magnetic field.
A) Differential rotation
B) Granules
C) The Zeeman effect
D) Spicules
E) The coronal hole
A) Differential rotation
B) Granules
C) The Zeeman effect
D) Spicules
E) The coronal hole

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
67
As the moon covers the solar disk during a solar eclipse, a flash spectrum of the sun s chromosphere can be recorded. This flash spectrum reveals an emission spectrum and provides information on the properties of the chromosphere. As the moon moves from the inner chromosphere to the outer chromosphere, the spectral lines present in the flash spectrum change. What is going on in the chromosphere that produces the changes in the flash spectrum?
I. The temperature of the chromosphere decreases as the distance from the photosphere increases.
II. The temperature of the chromosphere increases as the distance from the photosphere increases.
III. The density of the chromosphere decreases as the distance from the photosphere increases.
IV. The density of the chromosphere increases as the distance from the photosphere increases.
A) I,III
B) I, IV
C) II III
D) II IV
E) I
I. The temperature of the chromosphere decreases as the distance from the photosphere increases.
II. The temperature of the chromosphere increases as the distance from the photosphere increases.
III. The density of the chromosphere decreases as the distance from the photosphere increases.
IV. The density of the chromosphere increases as the distance from the photosphere increases.
A) I,III
B) I, IV
C) II III
D) II IV
E) I

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
68
The corona of the sun can be observed
A) during a lunar eclipse.
B) with a coronagraph.
C) using filtergrams.
D) a and b above
E) none of the above
A) during a lunar eclipse.
B) with a coronagraph.
C) using filtergrams.
D) a and b above
E) none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
69
What are the three layers of the Sun s atmosphere, in order of increasing distance from the surface?
A) Corona, chromosphere, photosphere
B) Photosphere, corona, chromosphere
C) Photosphere, chromosphere, corona
D) Chromosphere, photosphere, corona
A) Corona, chromosphere, photosphere
B) Photosphere, corona, chromosphere
C) Photosphere, chromosphere, corona
D) Chromosphere, photosphere, corona

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
70
A recent sunspot maximum occurred in 2001. What is the year of the sunspot maximum that immediately follows the 2001 maximum if the solar cycle continues?
A) 2006 or 2007
B) 2012
C) 2018
D) 2023
E) the last cycle started a Maunder minimum and the next maximum cannot be predicted.
A) 2006 or 2007
B) 2012
C) 2018
D) 2023
E) the last cycle started a Maunder minimum and the next maximum cannot be predicted.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
71
What is the explanation for the pattern of granulation seen on the visible surface of the Sun?
A) The granules form the base of a circulation pattern that extends from the photosphere to the outer corona.
B) The granules are regions of nuclear energy generation in the Sun s photosphere.
C) Each granule contains a strong magnetic field, which compresses and heats the gas underneath it.
D) The granules are the tops of hot gas that have risen from the Sun s convective zone.
A) The granules form the base of a circulation pattern that extends from the photosphere to the outer corona.
B) The granules are regions of nuclear energy generation in the Sun s photosphere.
C) Each granule contains a strong magnetic field, which compresses and heats the gas underneath it.
D) The granules are the tops of hot gas that have risen from the Sun s convective zone.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
72
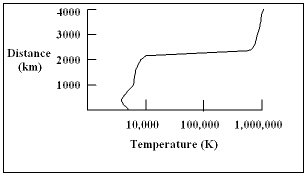 The diagram shows a plot of the temperature of the sun as a function of distance above the bottom of the photosphere. At what distance above the bottom of the photosphere does the temperature of the sun change the most rapidly with distance?
The diagram shows a plot of the temperature of the sun as a function of distance above the bottom of the photosphere. At what distance above the bottom of the photosphere does the temperature of the sun change the most rapidly with distance?A) 1,000 km
B) 2,300 km
C) 2,500 km to 4,000 km
D) 400 km
E) a and c

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
73
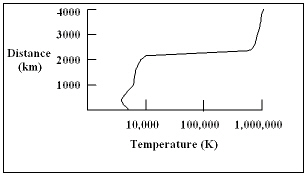 The diagram shows a plot of the temperature of the sun as a function of distance above the bottom of the photosphere. At what distance above the bottom of the photosphere is the temperature of the sun the smallest?
The diagram shows a plot of the temperature of the sun as a function of distance above the bottom of the photosphere. At what distance above the bottom of the photosphere is the temperature of the sun the smallest?A) 1000 km
B) 2300 km
C) 2500 km to 4000 km
D) 500 km
E) a and c

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
74
Sunspots are dark because
A) regions of the photosphere are obscured by material in the chromosphere.
B) shock waves move through the photosphere.
C) the sun rotates differentially.
D) the strong magnetic field inhibits the currents of hot gas rising from below.
E) they radiate their energy into space faster than the rest of the photosphere.
A) regions of the photosphere are obscured by material in the chromosphere.
B) shock waves move through the photosphere.
C) the sun rotates differentially.
D) the strong magnetic field inhibits the currents of hot gas rising from below.
E) they radiate their energy into space faster than the rest of the photosphere.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
75
The gas motions within granules on the solar surface are
A) upward in the centers of some cells and downward in others; the gas cools as it passes between individual granules.
B) actually motionless. The dark regions are absorption features from gases in the photosphere.
C) upward in the bright cell centers and downward around the darker edges.
D) downward in the bright cell centers and upward around the darker edges.
A) upward in the centers of some cells and downward in others; the gas cools as it passes between individual granules.
B) actually motionless. The dark regions are absorption features from gases in the photosphere.
C) upward in the bright cell centers and downward around the darker edges.
D) downward in the bright cell centers and upward around the darker edges.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
76
The intensity of a sunspot is found to be 3 times smaller than the intensity emitted by the solar surface. What is the approximate temperature of this sunspot if the temperature of the solar surface is 5800 K?
A) 4400 K
B) 470,000 K
C) 1900 K
D) 7600 K
E) 1400 K
A) 4400 K
B) 470,000 K
C) 1900 K
D) 7600 K
E) 1400 K

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
77
If the spectrum of a sunspot shows that it has a maximum intensity at 650 nm, what is the temperature of the sunspot?
A) 650 K
B) 5000 K
C) 1950 K
D) 4600 K
E) 10,000 K
A) 650 K
B) 5000 K
C) 1950 K
D) 4600 K
E) 10,000 K

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
78
Spicules
A) are found in the photosphere.
B) are magnetic disturbances that push large loops of material off the solar surface.
C) are responsible for twisting the solar magnetic field and causing the sunspot cycle.
D) appear in the corona near the north and south poles of the sun during a total solar eclipse.
E) are visible in filtergrams of the solar chromosphere.
A) are found in the photosphere.
B) are magnetic disturbances that push large loops of material off the solar surface.
C) are responsible for twisting the solar magnetic field and causing the sunspot cycle.
D) appear in the corona near the north and south poles of the sun during a total solar eclipse.
E) are visible in filtergrams of the solar chromosphere.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
79
A ____ is believed to occur when energy, stored in a twist in the solar magnetic field above a sunspot, is suddenly released.
A) solar flare
B) supergranule
C) spicule
D) coronal hole
E) none of the above
A) solar flare
B) supergranule
C) spicule
D) coronal hole
E) none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
80
The centers of granules
A) are hot material rising to the photosphere from below.
B) are cool material falling from the photosphere to the regions below.
C) are fainter and hotter than their surroundings.
D) are brighter and cooler than their surroundings.
E) show strong Zeeman effects.
A) are hot material rising to the photosphere from below.
B) are cool material falling from the photosphere to the regions below.
C) are fainter and hotter than their surroundings.
D) are brighter and cooler than their surroundings.
E) show strong Zeeman effects.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck


