Deck 2: Atoms and Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/45
Play
Full screen (f)
Deck 2: Atoms and Molecules
1
The Group II metals (Be, Mg, Ca, Sr, and Ba) are commonly referred to as the:
A) alkali metals
B) alkaline earth metals
C) halogen metals
D) lanthanide metals
A) alkali metals
B) alkaline earth metals
C) halogen metals
D) lanthanide metals
alkaline earth metals
2
Which pair of elements can be classified as noble gases?
A) H and He
B) Na and K
C) Kr and Ar
D) N and O
A) H and He
B) Na and K
C) Kr and Ar
D) N and O
Kr and Ar
3
A horizontal row of elements on the periodic table is referred to as a family.
False
4
Which of the following are examples of transition metals:
A) Fe and Zn
B) Sb and I
C) Pm and Gd
D) Al and Ga
A) Fe and Zn
B) Sb and I
C) Pm and Gd
D) Al and Ga

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
5
The nucleus is comprised of the atom's protons and electrons.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
6
The correct systematic name of AlCl3 is aluminum chloride.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
7
The average mass of an atom is determined by
A) taking a weighted average of all isotopic masses
B) averaging the masses of each isotope
C) taking a weighted average of all stable isotopic masses
D) adding the isotopic masses and dividing by the number of isotopes
A) taking a weighted average of all isotopic masses
B) averaging the masses of each isotope
C) taking a weighted average of all stable isotopic masses
D) adding the isotopic masses and dividing by the number of isotopes

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
8
Free radicals such as 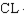 tend to be very reactive.
tend to be very reactive.
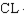 tend to be very reactive.
tend to be very reactive.
Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following elements are actinides?
A) Ti and Cr
B) U and Np
C) Sm and Er
D) Kr and Xe
A) Ti and Cr
B) U and Np
C) Sm and Er
D) Kr and Xe

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
10
The correct systematic name of V2O5 is divanadium pentoxide.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
11
All polymers form rigid solids at standard temperature and pressure.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
12
The most dense elements tend to be found in:
A) Period 2
B) Period 6
C) Period 5
D) Period 4
A) Period 2
B) Period 6
C) Period 5
D) Period 4

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
13
Pentane has a five carbon atom skeleton

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
14
CH3 − O − CH3 is an example of an alcohol

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
15
Elements in a periodic family tend to react in similar fashion.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
16
The chemical symbol for nitrite is NO31 − .

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
17
For a given family (ie. the alkali metals), the element in which period is most reactive?
A) 2nd
B) 3rd
C) 4th
D) 5th
A) 2nd
B) 3rd
C) 4th
D) 5th

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
18
Cations are particles with fewer electrons than protons.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
19
Gallium, arsenic, and silicon are used as metalloids in the semiconductor industry.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
20
Ions of opposite charges attract one another. This attraction is governed by:
A) Calbert's Law
B) Henry's Law
C) Franklin's Law
D) Coulomb's Law
A) Calbert's Law
B) Henry's Law
C) Franklin's Law
D) Coulomb's Law

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
21
The molecular formula of calcium phosphate is:
A) Ca3P2
B) CaPO3
C) Ca3(PO4)2
D) Ca2(PO4)3
A) Ca3P2
B) CaPO3
C) Ca3(PO4)2
D) Ca2(PO4)3

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
22
Consider 235U. How many neutrons are present in this nuclide?
A) 143
B) 235
C) 92
D) 177
A) 143
B) 235
C) 92
D) 177

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
23
The correct molecular formula for iron(II)bromide is:
A) FeBr3
B) FeBr2
C) I2Br2
D) IBr3
A) FeBr3
B) FeBr2
C) I2Br2
D) IBr3

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
24
How many electrons are present in a 40Ca2+ species?
A) 40
B) 38
C) 20
D) 18
A) 40
B) 38
C) 20
D) 18

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
25
The systematic (IUPAC) name of NaClO4 is:
A) sodium perchlorate
B) sodium chlorate
C) sodium hypochlorate
D) sodium chloride tetraoxide
A) sodium perchlorate
B) sodium chlorate
C) sodium hypochlorate
D) sodium chloride tetraoxide

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
26
The correct molecular formula of rubidium chloride is:
A) RuCl2
B) RbCl2
C) RuCl
D) RbCl
A) RuCl2
B) RbCl2
C) RuCl
D) RbCl

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
27
Which species contains 9 protons?
A) (16O)
B) (19F)
C) (35S)
D) (32P)
A) (16O)
B) (19F)
C) (35S)
D) (32P)

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
28
Consider the 203Hg. How many neutrons are present in this nuclide?
A) 203
B) 80
C) 283
D) 123
A) 203
B) 80
C) 283
D) 123

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
29
Alkali metal cations carry a charge of:
A) 1+
B) 2+
C) 2 −
D) 1 −
A) 1+
B) 2+
C) 2 −
D) 1 −

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
30
The correct molecular formula for ammonium chloride is:
A) NH3Cl
B) AmCl
C) NH4Cl
D) AmCl2
A) NH3Cl
B) AmCl
C) NH4Cl
D) AmCl2

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
31
If an ion contains 33 protons, 39 neutrons, and 34 electrons, the ion is:
A) (73Se1 − )
B) (72As1 − )
C) (67Y1+)
D) (73Se1+)
A) (73Se1 − )
B) (72As1 − )
C) (67Y1+)
D) (73Se1+)

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
32
The correct molecular formula of dinitrogen pentoxide is:
A) N2O5
B) (NO5)2
C) 2NO5
D) N2P5O
A) N2O5
B) (NO5)2
C) 2NO5
D) N2P5O

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
33
The correct molecular formula of tin(IV)oxide is:
A) TiO2
B) SnO2
C) SnO4
D) TiO4
A) TiO2
B) SnO2
C) SnO4
D) TiO4

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
34
Uranium, plutonium, and neptunium are classified as:
A) alkali metals
B) alkaline earth metals
C) lanthanides
D) actinides
A) alkali metals
B) alkaline earth metals
C) lanthanides
D) actinides

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
35
The systematic (IUPAC) name of MgI2 is:
A) manganese iodide
B) manganese diiodide
C) magnesium iodide
D) magnesium (II) iodide
A) manganese iodide
B) manganese diiodide
C) magnesium iodide
D) magnesium (II) iodide

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
36
CaCl2 is an example of a(n):
A) covalent compound
B) formula unit
C) molecular compound
D) organic acid
A) covalent compound
B) formula unit
C) molecular compound
D) organic acid

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
37
The systematic (IUPAC) name of CuO is:
A) copper (I) oxide
B) copper (II) oxide
C) copper oxide
D) copper (II) hydroxide
A) copper (I) oxide
B) copper (II) oxide
C) copper oxide
D) copper (II) hydroxide

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
38
The correct molecular formula for strontium chloride is:
A) SrCl2
B) StCl2
C) SrClO2
D) SrCl
A) SrCl2
B) StCl2
C) SrClO2
D) SrCl

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
39
The correct molecular formula for lead nitrate is:
A) PbN3
B) Pb(NO2)3
C) Pb(NO3)2
D) PNO3
A) PbN3
B) Pb(NO2)3
C) Pb(NO3)2
D) PNO3

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
40
The systematic (IUPAC) name of Sr(NO2)2 is:
A) strontium nitrate
B) strontium dinitrate
C) sirium nitrate
D) strontium nitrite
A) strontium nitrate
B) strontium dinitrate
C) sirium nitrate
D) strontium nitrite

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
41
The ____________________ provides a ratio of atoms in a compound.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
42
____________________ are nuclei having the same number of protons but different numbers of neutrons.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
43
The atomic mass of an atom is the number of ____________________ in that particular atom.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
44
The ratio of isotopes for a given element can be measured instrumentally using a(n) ____________________.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
45
When electrons are shared between pairs of atoms rather than donated from one atom to another or mobile across an entire lattice, a(n) ____________________ bond is present.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck


