Deck 4: Energy and Metabolism
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns
Question
Question
Match between columns
Question
Question
Question
Question
Question
Question
Question
Match between columns
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/80
Play
Full screen (f)
Deck 4: Energy and Metabolism
1
Which metabolite of alcohol is most toxic?
A) alcohol dehydrogenase
B) ethanol
C) acetaldehyde
D) acetate
E) ALDH
A) alcohol dehydrogenase
B) ethanol
C) acetaldehyde
D) acetate
E) ALDH
C
2
When ethanol (C2H5OH) and oxygen (O2) react together, they form carbon dioxide (CO2) and water (H2O). The resulting chemical reaction is: C 2 H 5 OH + 3O 2 → 2CO 2 + 3H 2 O
The coefficient in front of H2O indicates there are ____.
A) three oxygen atoms in the reaction
B) three carbon atoms in the water
C) three water molecules in the reaction
D) six water molecules in the reaction
E) six oxygen atoms in the reaction
The coefficient in front of H2O indicates there are ____.
A) three oxygen atoms in the reaction
B) three carbon atoms in the water
C) three water molecules in the reaction
D) six water molecules in the reaction
E) six oxygen atoms in the reaction
C
3
The energy in chemical bonds is what type of energy?
A) kinetic
B) potential
C) atomic
D) nuclear
E) thermal
A) kinetic
B) potential
C) atomic
D) nuclear
E) thermal
B
4
Which human organ is responsible for breaking down ethanol and other toxins?
A) stomach
B) liver
C) small intestine
D) pancreas
E) spleen
A) stomach
B) liver
C) small intestine
D) pancreas
E) spleen

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
5
What reaction does the enzyme alcohol dehydrogenase catalyze?
A) ethanol to acetate
B) ethanol to acetaldehyde
C) acetate to acetaldehyde
D) acetaldehyde to ethanol
E) acetaldehyde to acetate
A) ethanol to acetate
B) ethanol to acetaldehyde
C) acetate to acetaldehyde
D) acetaldehyde to ethanol
E) acetaldehyde to acetate

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
6
The energy that fuels most life on earth comes from ____.
A) the sun
B) heat
C) sucrose
D) water
E) glucose
A) the sun
B) heat
C) sucrose
D) water
E) glucose

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
7
Energy is defined as ____.
A) the capacity to do work
B) the movement of atoms and molecules
C) movement of electrons
D) measurement of food intake
E) capacity to store sugar
A) the capacity to do work
B) the movement of atoms and molecules
C) movement of electrons
D) measurement of food intake
E) capacity to store sugar

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
8
Long term, heavy drinking can lead to ____, a disease characterized by inflammation and destruction of the liver.
A) hepatoblastoma
B) gall stones
C) fatty liver disease
D) jaundice
E) cirrhosis
A) hepatoblastoma
B) gall stones
C) fatty liver disease
D) jaundice
E) cirrhosis

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
9
In a chemical reaction, if the reactants have more energy than the products, ____.
A) there will be a net release of energy
B) there will be a net loss of energy
C) the activation energy will be very high
D) the activation energy will be very low
E) more bonds were made than were broken
A) there will be a net release of energy
B) there will be a net loss of energy
C) the activation energy will be very high
D) the activation energy will be very low
E) more bonds were made than were broken

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
10
A cow converts the energy of glucose into the energy of ATP. What happens to most of the energy in that conversion?
A) It remains with the glucose-it can't be extracted.
B) It is lost as heat energy.
C) It is within the ATP molecule.
D) It is recycled back into glucose.
E) It is utilized in other metabolic pathways.
A) It remains with the glucose-it can't be extracted.
B) It is lost as heat energy.
C) It is within the ATP molecule.
D) It is recycled back into glucose.
E) It is utilized in other metabolic pathways.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is an example of the second law of thermodynamics?
A) c onversion of the energy in sunlight into glucose
B) t he use of gasoline to propel your car
C) ATP activation of a proton pump
D) a light bulb heats up after use
E) a nuclear reactor lights up the city
A) c onversion of the energy in sunlight into glucose
B) t he use of gasoline to propel your car
C) ATP activation of a proton pump
D) a light bulb heats up after use
E) a nuclear reactor lights up the city

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
12
The second law of thermodynamics states that ____.
A) the energy of the universe is a constant
B) energy can be neither created nor destroyed
C) energy disperses spontaneously
D) energy transformations create a more orderly universe
E) energy and matter are the same thing
A) the energy of the universe is a constant
B) energy can be neither created nor destroyed
C) energy disperses spontaneously
D) energy transformations create a more orderly universe
E) energy and matter are the same thing

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
13
What is the most likely source of various hangover symptoms?
A) acetate
B) acetaldehyde
C) ethanol
D) alcohol dehydrogenase
E) ALDH
A) acetate
B) acetaldehyde
C) ethanol
D) alcohol dehydrogenase
E) ALDH

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
14
A cirrhotic liver can no longer produce ____, which leads to swelling in the legs and abdomen. haptoglobin
A) alpha-fetoprotein
B) alcohol dehydrogenase
C) haptoglobin
D) fibronectin
E) albumin
A) alpha-fetoprotein
B) alcohol dehydrogenase
C) haptoglobin
D) fibronectin
E) albumin

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
15
If the activation energy for a chemical reaction is very high, the ____.
A) reaction will have an overall net gain of energy
B) reaction will have an overall net loss of energy
C) reaction will progress slowly
D) reaction will progress quickly
E) reactants have a lower energy than the products
A) reaction will have an overall net gain of energy
B) reaction will have an overall net loss of energy
C) reaction will progress slowly
D) reaction will progress quickly
E) reactants have a lower energy than the products

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
16
Energy flows in which pattern?
A) Sun → environment → consumers → producers
B) Sun → environment → producers → consumers
C) Sun → consumers → producers → environment
D) Sun → producers → consumers → environment
E) Sun→ producers → environment → consumers
A) Sun → environment → consumers → producers
B) Sun → environment → producers → consumers
C) Sun → consumers → producers → environment
D) Sun → producers → consumers → environment
E) Sun→ producers → environment → consumers

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
17
Energy ____.
A) can be created but not destroyed
B) cannot be created but can be destroyed
C) can be created and destroyed
D) cannot be created or destroyed
E) can be created using a particle accelerator
A) can be created but not destroyed
B) cannot be created but can be destroyed
C) can be created and destroyed
D) cannot be created or destroyed
E) can be created using a particle accelerator

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
18
Cells store energy in the form of ____.
A) sunlight
B) chloroplasts
C) carbon dioxide
D) glucose
E) water
A) sunlight
B) chloroplasts
C) carbon dioxide
D) glucose
E) water

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
19
The enzyme that is part of the metabolic pathway that breaks down alcohol is called ____.
A) alcohol dehydrogenase
B) alcohol oxidase
C) ethanol peroxidase
D) liver catalase
E) ethanol protease
A) alcohol dehydrogenase
B) alcohol oxidase
C) ethanol peroxidase
D) liver catalase
E) ethanol protease

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
20
Why does wood keep burning once it is lit?
A) Intermediates from the reaction drive the reaction forward.
B) Energy is given off during the reaction that drives the reaction forward.
C) The products have more energy than the reactants, therefore driving the reaction forward.
D) The reactants have more energy than the products, therefore driving the reaction forward.
E) The activation of the energy drives the reaction forward.
A) Intermediates from the reaction drive the reaction forward.
B) Energy is given off during the reaction that drives the reaction forward.
C) The products have more energy than the reactants, therefore driving the reaction forward.
D) The reactants have more energy than the products, therefore driving the reaction forward.
E) The activation of the energy drives the reaction forward.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is an example of an energy-requiring reaction?
A) The combustion of the gas in your car to move it forward.
B) The burning of a log to generate heat.
C) The synthesis of carbon dioxide into glucose.
D) The oxidation of metal to form rust.
E) The metabolism of glucose to form ATP.
A) The combustion of the gas in your car to move it forward.
B) The burning of a log to generate heat.
C) The synthesis of carbon dioxide into glucose.
D) The oxidation of metal to form rust.
E) The metabolism of glucose to form ATP.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
22
 Based on the given graph for the pH profile for GlyFa1, an enzyme used by a species of bacteria that lives in California copper mines, what is the optimal pH for GlyFa1 activity?
Based on the given graph for the pH profile for GlyFa1, an enzyme used by a species of bacteria that lives in California copper mines, what is the optimal pH for GlyFa1 activity?A) 0
B) 1
C) 2
D) 4
E) 6

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
23
Pepsin is an enzyme that functions in the stomach. Its optimum pH would be ____.
A) between 1 and 2
B) between 3 and 4
C) above 6
D) between 5 and 7.5
E) above 8.5
A) between 1 and 2
B) between 3 and 4
C) above 6
D) between 5 and 7.5
E) above 8.5

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
24
ATP contains ____.
A) alanine
B) arginine
C) phosphate
D) tyrosine
E) glucose
A) alanine
B) arginine
C) phosphate
D) tyrosine
E) glucose

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
25
A series of enzyme-mediated reactions by which cells build, remodel, or break down organic molecules is known as ____.
A) energy carriers
B) metabolic pathways
C) the induced-fit model
D) intermediary compounds
E) activation
A) energy carriers
B) metabolic pathways
C) the induced-fit model
D) intermediary compounds
E) activation

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
26
F. acidarmanus , a species of bacteria that lives in California copper mines, has adapted to its environment by altering the ____ at which its enzymes function.
A) temperature
B) pH
C) light levels
D) substrate concentration
E) salt concentration
A) temperature
B) pH
C) light levels
D) substrate concentration
E) salt concentration

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
27
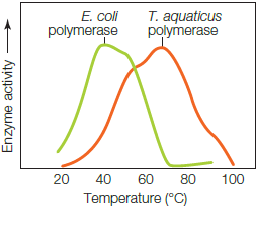 The graph shows the temperature profile for T. aquaticus polymerase, an enzyme used by a species of bacteria that lives in hot springs. What does the boxed region indicate?
The graph shows the temperature profile for T. aquaticus polymerase, an enzyme used by a species of bacteria that lives in hot springs. What does the boxed region indicate?A) The enzyme activity increases as substrates increase their kinetic energy.
B) The enzyme activity decreases as substrates increase their kinetic energy.
C) The enzyme activity increases as the enzyme denatures.
D) The enzyme activity decreases as the enzyme denatures.
E) The enzyme activity decreases as the enzyme refolds.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
28
If guncotton has a lower activation energy than gunpowder, then which is true about guncotton?
A) It is a highly explosive derivative of cholesterol.
B) It is used to make gunpowder.
C) It is less stable than gunpowder.
D) It is more stable than gunpowder.
E) It reacts less readily with oxygen than gunpowder.
A) It is a highly explosive derivative of cholesterol.
B) It is used to make gunpowder.
C) It is less stable than gunpowder.
D) It is more stable than gunpowder.
E) It reacts less readily with oxygen than gunpowder.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
29
Enzymes speed up a chemical reaction by ____.
A) lowering the activation energy
B) lowering the optimal pH
C) increasing the reaction temperature
D) increasing the energy in the system
E) increasing the activation energy
A) lowering the activation energy
B) lowering the optimal pH
C) increasing the reaction temperature
D) increasing the energy in the system
E) increasing the activation energy

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
30
Regulatory factors that bind to enzymes ____.
A) always increase enzyme activity
B) always decrease enzyme activity
C) alter the shape of the enzyme
D) alter the pH at which the enzyme works
E) alter the temperature at which the enzyme works
A) always increase enzyme activity
B) always decrease enzyme activity
C) alter the shape of the enzyme
D) alter the pH at which the enzyme works
E) alter the temperature at which the enzyme works

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
31
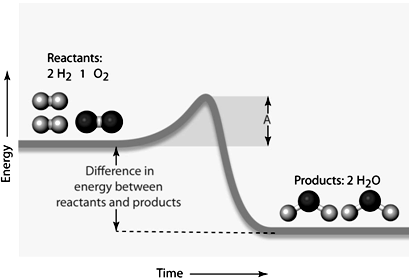 In the given figure, the amount of energy symbolized by the line "A" would be the ____.
In the given figure, the amount of energy symbolized by the line "A" would be the ____.A) reactant energy
B) product energy
C) cofactor energy
D) activation energy
E) intermediate energy

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
32
Most enzymes are composed of ____.
A) RNA only
B) protein only
C) substrate only
D) RNA and protein
E) protein and substrate
A) RNA only
B) protein only
C) substrate only
D) RNA and protein
E) protein and substrate

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
33
Why doesn't the gasoline in your car spontaneously ignite into flames? (The reaction of gasoline with oxygen is called a combustion reaction and is the reaction that is used to power your car.)
A) The products of a combustion reaction have more energy than the reactants.
B) The gasoline in your car is in an air-tight container, so it cannot react with the oxygen from the air.
C) The amount of energy in the bonds of the products exceeds the amount of energy in the bonds of the reactants.
D) The reactants of the combustion of gasoline have less energy and the products.
E) The gasoline in your car alone cannot overcome the activation energy.
A) The products of a combustion reaction have more energy than the reactants.
B) The gasoline in your car is in an air-tight container, so it cannot react with the oxygen from the air.
C) The amount of energy in the bonds of the products exceeds the amount of energy in the bonds of the reactants.
D) The reactants of the combustion of gasoline have less energy and the products.
E) The gasoline in your car alone cannot overcome the activation energy.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the ADH reaction and answer the following question. ADH stands for alcohol dehydrogenase. 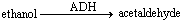 In this reaction, ADH represents the ____.
In this reaction, ADH represents the ____.
A) enzyme
B) reactant
C) product
D) activation energy
E) trigger
 In this reaction, ADH represents the ____.
In this reaction, ADH represents the ____.A) enzyme
B) reactant
C) product
D) activation energy
E) trigger

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
35
The minimum amount of energy needed to get a chemical reaction started is called the ____ energy.
A) activation
B) reaction
C) enzymatic
D) chemical
E) triggering
A) activation
B) reaction
C) enzymatic
D) chemical
E) triggering

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
36
Substances that enter a reaction are termed ____.
A) intermediates
B) enzymes
C) energy carriers
D) reactants
E) end products
A) intermediates
B) enzymes
C) energy carriers
D) reactants
E) end products

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the ADH reaction and answer the following question. ADH stands for alcohol dehydrogenase. 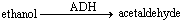 In this reaction, acetaldehyde represents the ____.
In this reaction, acetaldehyde represents the ____.
A) enzyme
B) reactant
C) product
D) activation energy
E) trigger
 In this reaction, acetaldehyde represents the ____.
In this reaction, acetaldehyde represents the ____.A) enzyme
B) reactant
C) product
D) activation energy
E) trigger

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
38
The fact that the earth does not go up in flames in spite of the richness of oxygen in our environment is related to which of the following concepts?
A) the activation energy needed to break bonds
B) the fact that reactants always have less energy than products
C) the first law of thermodynamics
D) the second law of thermodynamics
E) the abundance of water in our atmosphere, which inhibits combustion
A) the activation energy needed to break bonds
B) the fact that reactants always have less energy than products
C) the first law of thermodynamics
D) the second law of thermodynamics
E) the abundance of water in our atmosphere, which inhibits combustion

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
39
When a protein denatures, it ____.
A) changes shape
B) changes temperature
C) changes pH
D) binds more efficiently to its cofactor
E) catalyzes reactions more efficiently
A) changes shape
B) changes temperature
C) changes pH
D) binds more efficiently to its cofactor
E) catalyzes reactions more efficiently

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
40
Consider the ADH reaction and answer the following question. ADH stands for alcohol dehydrogenase. 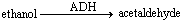 In the given reaction, ethanol represents the ____.
In the given reaction, ethanol represents the ____.
A) enzyme
B) reactant
C) product
D) activation energy
E) trigger
 In the given reaction, ethanol represents the ____.
In the given reaction, ethanol represents the ____.A) enzyme
B) reactant
C) product
D) activation energy
E) trigger

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
41
Oxygen, carbon dioxide, and other small molecules cross the plasma membrane through the process of ____.
A) diffusion
B) osmosis
C) endocytosis and exocytosis
D) active transport
E) facilitated diffusion
A) diffusion
B) osmosis
C) endocytosis and exocytosis
D) active transport
E) facilitated diffusion

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
42
Heating up a reaction increases the speed of a reaction until the ____.
A) enzyme denatures
B) heat changes the pH of the reaction
C) maximum speed is achieved
D) cofactors can ' t bind the enzyme
E) reaction begins to experience feedback inhibition
A) enzyme denatures
B) heat changes the pH of the reaction
C) maximum speed is achieved
D) cofactors can ' t bind the enzyme
E) reaction begins to experience feedback inhibition

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
43
Unlike plants, fungi, and bacteria, animal cells cannot resist volume increases in hypotonic environments. This is because unlike those other organisms, animal cells ____.
A) do not have central vacuoles
B) do not have cell walls
C) do not have pumps that can actively pump out excess fluid
D) are smaller than those other cells and thus cannot handle large increases in volume
E) have relatively more solutes
A) do not have central vacuoles
B) do not have cell walls
C) do not have pumps that can actively pump out excess fluid
D) are smaller than those other cells and thus cannot handle large increases in volume
E) have relatively more solutes

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
44
Passive molecular diffusion occurs when ____.
A) the energy of ATP is added
B) random collisions between molecules occur
C) there are variations in molecular sizes
D) enzymes catalyze their movement
E) vesicles break off from the membrane
A) the energy of ATP is added
B) random collisions between molecules occur
C) there are variations in molecular sizes
D) enzymes catalyze their movement
E) vesicles break off from the membrane

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
45
Wilting of a plant occurs ____.
A) if the plant is placed in an isotonic solution
B) if there is a rise in turgor pressure
C) as a result of facilitated diffusion
D) if the plant is placed in a hypertonic solution
E) if the plant is placed in a hypotonic solution
A) if the plant is placed in an isotonic solution
B) if there is a rise in turgor pressure
C) as a result of facilitated diffusion
D) if the plant is placed in a hypertonic solution
E) if the plant is placed in a hypotonic solution

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
46
Osmotic pressure is to a plant cell as ____ is to a ____.
A) air; balloon
B) water; water glass
C) fire; campfire
D) wind; windmill
E) dirt; vacuum cleaner
A) air; balloon
B) water; water glass
C) fire; campfire
D) wind; windmill
E) dirt; vacuum cleaner

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
47
Osmosis involves the movement of ____ across a semi-permeable membrane from an area of ____.
A) solutes; high solute concentration to an area of low solute concentration
B) water; high solute concentration to an area of low solute concentration
C) solvents; high solute concentration to an area of low solute concentration
D) solutes; low solute concentration to an area of high solute concentration
E) water; low solute concentration to an area of high solute concentration
A) solutes; high solute concentration to an area of low solute concentration
B) water; high solute concentration to an area of low solute concentration
C) solvents; high solute concentration to an area of low solute concentration
D) solutes; low solute concentration to an area of high solute concentration
E) water; low solute concentration to an area of high solute concentration

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
48
The amount of turgor that is enough to stop osmosis is called ____.
A) the wilting point
B) osmotic pressure
C) hypotonicity
D) expansion pressure
E) hypertonicity
A) the wilting point
B) osmotic pressure
C) hypotonicity
D) expansion pressure
E) hypertonicity

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
49
NAD+ is considered a(n) ____.
A) enzyme
B) coenzyme
C) regulatory molecule
D) active site
E) intermediate
A) enzyme
B) coenzyme
C) regulatory molecule
D) active site
E) intermediate

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
50
Diffusion of water from a hypertonic solution to a hypotonic solution across a semi-permeable membrane ____.
A) will occur until both solutions are isotonic
B) will occur until both sides are hypotonic
C) will occur until both sides are hypertonic
D) will occur until the tonicities are reversed
E) will not occur
A) will occur until both solutions are isotonic
B) will occur until both sides are hypotonic
C) will occur until both sides are hypertonic
D) will occur until the tonicities are reversed
E) will not occur

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
51
A concentration gradient ceases to exist when ____.
A) all molecules have moved from low concentration to high concentration
B) the membrane pores close
C) the temperature drops
D) there is equilibrium between the two sides of a membrane
E) bulk flow intervenes
A) all molecules have moved from low concentration to high concentration
B) the membrane pores close
C) the temperature drops
D) there is equilibrium between the two sides of a membrane
E) bulk flow intervenes

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
52
Which solution has the potential to diffuse the fastest?
A) A solution with large molecules as opposed to smaller ones.
B) A solution with a higher concentration of molecules as opposed to one with a lower concentration.
C) A solution that is under low pressure as opposed to one under high pressure.
D) A solution that is at a low temperature as opposed to one at a higher temperature.
E) A solution containing a hydrophilic solute as opposed to a salt.
A) A solution with large molecules as opposed to smaller ones.
B) A solution with a higher concentration of molecules as opposed to one with a lower concentration.
C) A solution that is under low pressure as opposed to one under high pressure.
D) A solution that is at a low temperature as opposed to one at a higher temperature.
E) A solution containing a hydrophilic solute as opposed to a salt.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following occurs in feedback inhibition?
A) Coenzymes block enzyme activity.
B) Products of metabolic reactions block enzyme activity.
C) NADH is altered in electron transport chains.
D) ADP is phosphorylated.
E) Low reactant concentrations decrease enzyme activity.
A) Coenzymes block enzyme activity.
B) Products of metabolic reactions block enzyme activity.
C) NADH is altered in electron transport chains.
D) ADP is phosphorylated.
E) Low reactant concentrations decrease enzyme activity.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
54
A substrate is another term for a(n) ____.
A) enzyme
B) product
C) reactant
D) active site
E) cofactor
A) enzyme
B) product
C) reactant
D) active site
E) cofactor

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
55
An enzyme's specificity is based on ____.
A) the shape of its active site
B) the amount of activation energy it requires
C) the number of amino acids in its structure
D) the nature of its cofactors
E) the conformation of the products
A) the shape of its active site
B) the amount of activation energy it requires
C) the number of amino acids in its structure
D) the nature of its cofactors
E) the conformation of the products

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
56
What happens to a molecule when it is phosphorylated?
A) It loses energy.
B) It receives a phosphate group.
C) It becomes denatured.
D) It loses electrons and protons.
E) It gains electrons.
A) It loses energy.
B) It receives a phosphate group.
C) It becomes denatured.
D) It loses electrons and protons.
E) It gains electrons.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
57
The net direction that an ion or molecule moves is ____.
A) dependent upon the size of the molecule
B) unpredictable because the movement is random
C) the result of concentration differences
D) controlled by the temperature of the medium
E) controlled by the membranes in the vicinity
A) dependent upon the size of the molecule
B) unpredictable because the movement is random
C) the result of concentration differences
D) controlled by the temperature of the medium
E) controlled by the membranes in the vicinity

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
58
A metabolic reaction will most likely reverse itself when the ____.
A) pH is too high
B) temperature is too low
C) reactant concentration is too high
D) product concentration is too high
E) enzyme concentration is too high
A) pH is too high
B) temperature is too low
C) reactant concentration is too high
D) product concentration is too high
E) enzyme concentration is too high

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
59
If a single-celled freshwater organism is transferred to saltwater, which of the following is likely to happen?
A) The cell will burst.
B) The cell will shrink.
C) Salt will be pumped out of the cell.
D) Salt will be pumped into the cell.
E) Enzymes will flow out of the cell.
A) The cell will burst.
B) The cell will shrink.
C) Salt will be pumped out of the cell.
D) Salt will be pumped into the cell.
E) Enzymes will flow out of the cell.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
60
What happens in an electron transport chain?
A) Electrons move from a low energy level to a higher energy level.
B) Electron movement transfers energy to enzymes and other molecules.
C) Electron movement generates energy during each step.
D) Energy from the electrons is stored for future use in pigments.
E) The energy from the electron movement is used to break down ATP.
A) Electrons move from a low energy level to a higher energy level.
B) Electron movement transfers energy to enzymes and other molecules.
C) Electron movement generates energy during each step.
D) Energy from the electrons is stored for future use in pigments.
E) The energy from the electron movement is used to break down ATP.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
61
The process by which a cell takes in a small amount of extracellular fluid and its contents by the ballooning inward of the plasma membrane is the definition of ____.
A) endocytosis
B) exocytosis
C) phagocytosis
D) passive transport
E) osmosis
A) endocytosis
B) exocytosis
C) phagocytosis
D) passive transport
E) osmosis

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
62
Once glucose has entered a cell, what prevents it from diffusing back out of the cell?
A) Glucose pumps actively transported any leaked glucose back into the cell.
B) One side of the cell membrane is permeable to glucose, but the other side is not.
C) Glucose inhibitors attach to glucose once it's in the cell.
D) Glucose is phosphorylated, preventing it from leaving the cell.
E) Glucose is hydrolyzed into two three-carbon molecules that cannot diffuse in a reverse direction.
A) Glucose pumps actively transported any leaked glucose back into the cell.
B) One side of the cell membrane is permeable to glucose, but the other side is not.
C) Glucose inhibitors attach to glucose once it's in the cell.
D) Glucose is phosphorylated, preventing it from leaving the cell.
E) Glucose is hydrolyzed into two three-carbon molecules that cannot diffuse in a reverse direction.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
63
To engulf a bacterium, a white blood cell would use ____.
A) facilitated diffusion
B) osmosis
C) phagocytosis
D) exocytosis
E) sodium-potassium pumps
A) facilitated diffusion
B) osmosis
C) phagocytosis
D) exocytosis
E) sodium-potassium pumps

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
64
The sodium - potassium pump is an example of ____.
A) facilitated diffusion
B) simple diffusion
C) osmosis
D) active transport
E) bulk flow
A) facilitated diffusion
B) simple diffusion
C) osmosis
D) active transport
E) bulk flow

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
65
Match between columns

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
66
Movement of substances that requires the expenditure of ATP molecules is called ____.
A) facilitated diffusion
B) simple diffusion
C) osmosis
D) active transport
E) bulk flow
A) facilitated diffusion
B) simple diffusion
C) osmosis
D) active transport
E) bulk flow

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
67
Match between columns

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
68
The initial event that triggers phagocytosis is when the ____.
A) solute concentration is higher inside the cell than outside
B) solute concentrations is lower inside the cell than outside
C) target protein binds to the receptor
D) enzymes bind to the membrane
E) vesicles bud off from the membrane
A) solute concentration is higher inside the cell than outside
B) solute concentrations is lower inside the cell than outside
C) target protein binds to the receptor
D) enzymes bind to the membrane
E) vesicles bud off from the membrane

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
69
Order the steps of exocytosis: 1. The vesicle membrane fuses with the plasma membrane.
2. The vesicle moves to the plasma membrane.
3. The vesicles contents are released to the extracellular fluid.
A) 1 → 2 → 3
B) 1 → 3 → 2
C) 2 → 1 → 3
D) 2 → 3 → 1
E) 3 → 1 → 2
2. The vesicle moves to the plasma membrane.
3. The vesicles contents are released to the extracellular fluid.
A) 1 → 2 → 3
B) 1 → 3 → 2
C) 2 → 1 → 3
D) 2 → 3 → 1
E) 3 → 1 → 2

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
70
When a phosphate group is transferred from ATP, active transport pumps ____.
A) trigger exocytosis
B) change their shapes
C) become ionized
D) fuse with the surrounding phospholipid membrane
E) are destroyed
A) trigger exocytosis
B) change their shapes
C) become ionized
D) fuse with the surrounding phospholipid membrane
E) are destroyed

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
71
Osmosis is an example of ____.
A) facilitated diffusion
B) passive transport
C) active transport
D) exocytosis
E) endocytosis
A) facilitated diffusion
B) passive transport
C) active transport
D) exocytosis
E) endocytosis

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
72
How do sodium-potassium pumps work?
A) Sodium is pumped into the cell and potassium diffuses out.
B) Sodium is pumped out of the cell and potassium diffuses in.
C) Sodium and potassium are both pumped into the cell simultaneously.
D) Sodium and potassium are both pumped out of the cell simultaneously.
E) Sodium only binds to the pump to make it receptive to potassium which then is pumped into the cell.
A) Sodium is pumped into the cell and potassium diffuses out.
B) Sodium is pumped out of the cell and potassium diffuses in.
C) Sodium and potassium are both pumped into the cell simultaneously.
D) Sodium and potassium are both pumped out of the cell simultaneously.
E) Sodium only binds to the pump to make it receptive to potassium which then is pumped into the cell.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
73
A solute passively diffuses through a transport protein out of the cell, therefore, ____.
A) the concentration of the solute is higher inside the cell than outside
B) the concentration of the solute is lower inside the cell than outside
C) the concentration of the solute is the same inside and outside the cell
D) the concentration of water is higher inside the cell than outside
E) the concentration of water is lower inside the cell than outside
A) the concentration of the solute is higher inside the cell than outside
B) the concentration of the solute is lower inside the cell than outside
C) the concentration of the solute is the same inside and outside the cell
D) the concentration of water is higher inside the cell than outside
E) the concentration of water is lower inside the cell than outside

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
74
Match between columns

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
75
Glucose is transported into a cell by ____.
A) active transport
B) exocytosis
C) facilitated diffusion
D) phagocytosis
E) osmosis
A) active transport
B) exocytosis
C) facilitated diffusion
D) phagocytosis
E) osmosis

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
76
Red blood cells contain 2 percent salt. What will happen when the cell is placed in a 10 percent salt solution?
A) The cell will burst.
B) The cell will shrink.
C) The cell will remain unchanged, but salt will diffuse out of the cell.
D) The cell will remain unchanged, but salt will diffuse into the cell.
E) The cell will remain unchanged, but water will diffuse out of the cell.
A) The cell will burst.
B) The cell will shrink.
C) The cell will remain unchanged, but salt will diffuse out of the cell.
D) The cell will remain unchanged, but salt will diffuse into the cell.
E) The cell will remain unchanged, but water will diffuse out of the cell.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
77
Active transport pumps typically move solutes from areas of ____ concentration to areas of ____ concentration.
A) high; low
B) low; high
C) neutral; high
D) neutral; low
E) low; neutral
A) high; low
B) low; high
C) neutral; high
D) neutral; low
E) low; neutral

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
78
A plant wilts when ____.
A) the soil water becomes hypotonic with respect to the cytoplasm
B) the soil water becomes hypertonic with respect to the cytoplasm
C) the turgor inside the cell increases
D) the turgor in the soil disappears
E) osmotic pressure is equal to the turgor pressure
A) the soil water becomes hypotonic with respect to the cytoplasm
B) the soil water becomes hypertonic with respect to the cytoplasm
C) the turgor inside the cell increases
D) the turgor in the soil disappears
E) osmotic pressure is equal to the turgor pressure

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
79
Calcium ion concentrations are tightly regulated because of the importance of calcium in biological functions. Calcium ion concentrations are ____ inside the cell than/as they are outside the cell.
A) slightly higher
B) slightly lower
C) the same
D) much higher
E) much lower
A) slightly higher
B) slightly lower
C) the same
D) much higher
E) much lower

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
80
Transporting a solute against its concentration gradient requires ____.
A) energy
B) a gated channel
C) exocytosis
D) hypotonic and hypertonic differences across the cell membrane
E) vesicle formation
A) energy
B) a gated channel
C) exocytosis
D) hypotonic and hypertonic differences across the cell membrane
E) vesicle formation

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck


