Deck 7: Chemical Bonding and Molecular Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/45
Play
Full screen (f)
Deck 7: Chemical Bonding and Molecular Structure
1
The bond angles of a tetrahedron are 120°.
False
2
Which of the following is the correct charge for a bromine anion?
A)1+
B)1 −
C)0
D)2 −
A)1+
B)1 −
C)0
D)2 −
1 −
3
In the context of the formation of cations, large jumps in ionization energy occur whenever an electron removal disrupts an electron configuration ending in _____.
A)ns2
B)ns1
C)np6
D)np4
A)ns2
B)ns1
C)np6
D)np4
np6
4
An ionic bond is formed by the electrostatic attraction of oppositely charged ions.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
5
Chemical species are said to be isoelectronic if they have the same Lewis structure (regardless of charge). In order to achieve an electron configuration that is isoelectric with a noble gas, nonmetals tend to _____.
A)gain electrons
B)lose electrons
C)form stable free radicals
D)both gain electrons and form stable free radicals
A)gain electrons
B)lose electrons
C)form stable free radicals
D)both gain electrons and form stable free radicals

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
6
When two s orbitals approach one another and overlap side to side, they form a pi bond .

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
7
Alkali metals tend to form cations.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following elements favors forming covalent bonds ?
A)Na
B)K
C)C
D)Ca
A)Na
B)K
C)C
D)Ca

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
9
The most stable rubidium cation has a charge of _____.
A)3+
B)2+
C)1 −
D)1+
A)3+
B)2+
C)1 −
D)1+

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
10
Halogens tend to form anions.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
11
Ionic bonds are based on the sharing of pairs of electrons between two atoms .

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
12
The valence shell electron pair repulsion (VSEPR)theory states that molecules assume a shape that allows them to minimize the repulsions between electron pairs in the valence shell of the central atom .

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
13
Among the following substances, which is the most reactive ?
A)SiO2
B)Sr(NO3)2
C)Cl2
D)C6H12O6
A)SiO2
B)Sr(NO3)2
C)Cl2
D)C6H12O6

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
14
In resonance structures, the positions of all electrons are identical; only the positions of the atoms are different .

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
15
Widely separated atoms do not interact but will reach an optimal separation distance called the bond length and form a bond.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
16
The most stable aluminum cation has a charge of _____.
A)3+
B)2+
C)1 −
D)1+
A)3+
B)2+
C)1 −
D)1+

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
17
The energy required to break a covalent bond is referred to as ionization energy .

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following electron configurations belongs to an atom that is most likely to be involved in an ionic bond?
A)1s22s22p63s2
B)1s22s22p6
C)1s22s22p63s23p3
D)1s22s22p63s23p6
A)1s22s22p63s2
B)1s22s22p6
C)1s22s22p63s23p3
D)1s22s22p63s23p6

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following electron configurations belongs to an atom that is most likely to be involved in a covalent bond?
A)1s22s22p63s2
B)1s22s22p6
C)1s22s22p63s23p3
D)1s22s22p63s23p6
A)1s22s22p63s2
B)1s22s22p6
C)1s22s22p63s23p3
D)1s22s22p63s23p6

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following elements can most easily form a cation ?
A)He
B)Na
C)Si
D)P
A)He
B)Na
C)Si
D)P

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
21
The assignment of electrons in Lewis dot structures is limited to :
A)the "p" sub-orbital.
B)valence electrons.
C)the "d" sub-orbital.
D)only those in the first group.
A)the "p" sub-orbital.
B)valence electrons.
C)the "d" sub-orbital.
D)only those in the first group.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
22
According to Coulomb's law, the force between two charges :
A)increases with the magnitude of charges.
B)decreases with the magnitude of charges.
C)does not depend on the magnitude of charges.
D)becomes quantum mechanically unstable at absolute zero temperature.
A)increases with the magnitude of charges.
B)decreases with the magnitude of charges.
C)does not depend on the magnitude of charges.
D)becomes quantum mechanically unstable at absolute zero temperature.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
23
What is the electron configuration of the calcium cation?
A)1s22s22p63s23p64s1
B)1s22s22p63s13p64s2
C)1s22s22p63s23p6
D)1s22s22p63s23p63d10
A)1s22s22p63s23p64s1
B)1s22s22p63s13p64s2
C)1s22s22p63s23p6
D)1s22s22p63s23p63d10

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
24
Multiple bonding results from the sharing of more than one electron pair. An example of this phenomenon is the double bond. In this case, the second bond is known as a _____.
A)second single bond
B)polar bond
C)sigma bond
D)pi bond
A)second single bond
B)polar bond
C)sigma bond
D)pi bond

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
25
According to the valence shell electron pair repulsion theory, which molecule shape best describes the geometry of ammonia ?
A)Octahedral
B)Square planar
C)Trigonal planar
D)Trigonal pyramidal
A)Octahedral
B)Square planar
C)Trigonal planar
D)Trigonal pyramidal

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
26
If the combined radii of each pair is assumed equal, which pair of atoms releases the most energy during the formation of an ion pair?
A)Na and Br
B)Al and S
C)Rb and F
D)K and O
A)Na and Br
B)Al and S
C)Rb and F
D)K and O

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following molecules has the lowest polarity ?
A)KI
B)C2H6
C)Ce(NO3)3
D)H2O
A)KI
B)C2H6
C)Ce(NO3)3
D)H2O

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
28
According to the valence shell electron pair repulsion theory, which molecule shape best describes the geometry of SF 6 ?
A)Octahedral
B)Square planar
C)Square pyramidal
D)Tetrahedral
A)Octahedral
B)Square planar
C)Square pyramidal
D)Tetrahedral

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
29
Draw the Lewis dot structure of nitrate. How many resonance states are possible?
A)3
B)2
C)1
D)0
A)3
B)2
C)1
D)0

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
30
There are _____ valence electrons available for the Lewis dot structure for 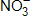 .
.
A)23
B)24
C)27
D)20
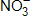 .
.A)23
B)24
C)27
D)20

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following compounds illustrates sp3hybridization?
A)C2H4
B)CH4
C)C2H2
D)V2O5
A)C2H4
B)CH4
C)C2H2
D)V2O5

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following has the largest ionic radius?
A)Al3+
B)I −
C)S
D)K+
A)Al3+
B)I −
C)S
D)K+

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
33
The symbol for chloride ion is _____.
A)Cl -
B)Cl2
C)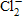
D)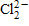
A)Cl -
B)Cl2
C)
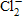
D)
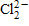

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following has the largest radius?
A)Na +
B)K+
C)Rb +
D)Cs+
A)Na +
B)K+
C)Rb +
D)Cs+

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following has the largest radius?
A)I −
B)Br
C)Cl −
D)F
A)I −
B)Br
C)Cl −
D)F

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following statements is true of covalent bonds ?
A)They mostly involve interactions between alkali metals and halogens.
B)They are formed when electrons from one atom are transferred to another atom.
C)They involve a cationic rearrangement of atoms.
D)They are formed when a significant electron density builds up between two nuclei.
A)They mostly involve interactions between alkali metals and halogens.
B)They are formed when electrons from one atom are transferred to another atom.
C)They involve a cationic rearrangement of atoms.
D)They are formed when a significant electron density builds up between two nuclei.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
37
Which hybridization best characterizes the C atom involved in a CH4molecule?
A)sp
B)sp2
C)sp3
D)sp4
A)sp
B)sp2
C)sp3
D)sp4

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following molecules has the highest polarity ?
A)NaCl
B)C2H6
C)HF
D)H2O
A)NaCl
B)C2H6
C)HF
D)H2O

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following illustrates the electron configuration of Ca 2 + ?
A)1s22s22p63s23p6
B)1s22s22p63s23p64s2
C)1s22s22p63s23p63d10
D)1s22s22p6
A)1s22s22p63s23p6
B)1s22s22p63s23p64s2
C)1s22s22p63s23p63d10
D)1s22s22p6

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
40
According to the valence shell electron pair repulsion theory, what is the molecular geometry of PCl 3 ?
A)Trigonal pyramidal
B)See-saw
C)Tetrahedral
D)T-shape
A)Trigonal pyramidal
B)See-saw
C)Tetrahedral
D)T-shape

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
41
The greater the difference in electronegativity, the less polar the bond .

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
42
Electronegativity is an energy change that can be determined experimentally .

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
43
According to the valence shell electron repulsion theory, a CH 4 molecule assumes the shape of a _____.
A)tetrahedron
B)triangle
C)straight line
D)trigonal pyramid
A)tetrahedron
B)triangle
C)straight line
D)trigonal pyramid

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
44
Small, highly charged ions tend to form i onic compounds with large lattice energies .

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
45
How many resonance structures can be drawn for 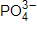 ?
?
A)1
B)2
C)3
D)4
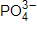 ?
?A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck


