Deck 1: Molecular Reasons
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/66
Play
Full screen (f)
Deck 1: Molecular Reasons
1
Thales believed that _____ is the principle element of all things.
A)earth
B)air
C)fire
D)water
E)gold
A)earth
B)air
C)fire
D)water
E)gold
water
2
The predecessor to chemistry is known as ____.
A)alchemy
B)pre-chemistry
C)biology
D)biochemistry
E)physical science
A)alchemy
B)pre-chemistry
C)biology
D)biochemistry
E)physical science
alchemy
3
Which of these is the best definition of a scientific law?
A)A prediction based on a limited number of observations.
B)A method of explaining observations that appear contradictory.
C)A broadly applicable generalization with virtually no exceptions.
D)A method for approaching problems that is used by all scientists.
E)A rule made by scientists to ensure consistency in their observations.
A)A prediction based on a limited number of observations.
B)A method of explaining observations that appear contradictory.
C)A broadly applicable generalization with virtually no exceptions.
D)A method for approaching problems that is used by all scientists.
E)A rule made by scientists to ensure consistency in their observations.
A broadly applicable generalization with virtually no exceptions.
4
Which two scientists are credited with the endorsement of a sun centered universe?
A)Dalton and Plato
B)Boyle and Copernicus
C)Copernicus and Galileo
D)Democritus and Vesalius
E)Copernicus and Vesalius
A)Dalton and Plato
B)Boyle and Copernicus
C)Copernicus and Galileo
D)Democritus and Vesalius
E)Copernicus and Vesalius

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
5
Which of these statements is correct ?
A)The amount of carbon on earth is essentially constant.
B)The amount of carbon on earth fluctuates with the seasons.
C)The amount of carbon on earth is increasing due to plant and animal growth.
D)The amount of carbon on earth is decreasing due to consumption of carbon based fuels.
E)Both B and C are correct.
A)The amount of carbon on earth is essentially constant.
B)The amount of carbon on earth fluctuates with the seasons.
C)The amount of carbon on earth is increasing due to plant and animal growth.
D)The amount of carbon on earth is decreasing due to consumption of carbon based fuels.
E)Both B and C are correct.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
6
The French chemist Antoine Lavoisier found that the weight of objects before burning and the weight of the products after burning were equal. He concluded that the total weight did not change during a process. Which of these best describes Lavoisier's conclusion?
A)From observation, Lavoisier created a scientific law.
B)From observation, Lavoisier created a scientific theory.
C)From scientific law, Lavoisier created a scientific theory.
D)From experimentation, Lavoisier created a scientific law.
E)From observation, Lavoisier created a scientific conclusion.
A)From observation, Lavoisier created a scientific law.
B)From observation, Lavoisier created a scientific theory.
C)From scientific law, Lavoisier created a scientific theory.
D)From experimentation, Lavoisier created a scientific law.
E)From observation, Lavoisier created a scientific conclusion.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
7
A statement which summarizes the data obtained from a series of observations is known as a(n)____.
A)observation
B)law
C)theory
D)conclusion
E)experiment
A)observation
B)law
C)theory
D)conclusion
E)experiment

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
8
Which of these is the best definition of matter?
A)The pull of gravity on an object.
B)Anything that has weight and volume.
C)Anything that has mass and occupies space.
D)Anything that is directly proportional to weight.
E)The measure of the amount of space an object occupies.
A)The pull of gravity on an object.
B)Anything that has weight and volume.
C)Anything that has mass and occupies space.
D)Anything that is directly proportional to weight.
E)The measure of the amount of space an object occupies.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
9
To explain natural phenomena scientists must _____.
A)have an opinion
B)make observations
C)guess correctly most of the time
D)agree with existing theories
A)have an opinion
B)make observations
C)guess correctly most of the time
D)agree with existing theories

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
10
Which of these contributions did Alchemists of the Middle Ages make to modern science?
A)Sun centered universe and metallurgy
B)Metallurgy and development of scientific techniques
C)Scientific method and the Law of Conservation of Mass
D)Law of Conservation of Matter and The First Atomic Theory
E)Law of Conservation of Mass and the Law of Constant Composition
A)Sun centered universe and metallurgy
B)Metallurgy and development of scientific techniques
C)Scientific method and the Law of Conservation of Mass
D)Law of Conservation of Matter and The First Atomic Theory
E)Law of Conservation of Mass and the Law of Constant Composition

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
11
Which of these statements about the scientific method is incorrect ?
A)It is a group of absolute truths.
B)It uses experiments that are reproducible
C)It is used for testing claims about the natural world.
D)It requires one to propose a theory and perform experiments to give results which confirm or disclaim the theory.
E)All of these are correct statements.
A)It is a group of absolute truths.
B)It uses experiments that are reproducible
C)It is used for testing claims about the natural world.
D)It requires one to propose a theory and perform experiments to give results which confirm or disclaim the theory.
E)All of these are correct statements.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
12
Identify the entities which will correctly complete the flow chart.
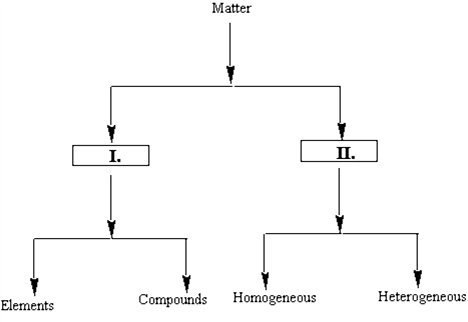

A)I
B)II
C)III
D)IV
E)V


A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
13
Which scientist is incorrectly matched with his idea or theory? 
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
14
A(n)____ is an explanation of a scientific observation.
A)law
B)theory
C)conclusion
D)prediction
E)epiphany
A)law
B)theory
C)conclusion
D)prediction
E)epiphany

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
15
Which of these is not part of the scientific method?
A)Observation
B)Law
C)Theory
D)Conclusion
E)Experiment
A)Observation
B)Law
C)Theory
D)Conclusion
E)Experiment

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
16
Which of these is the best definition of a scientific theory?
A)A prediction based on a limited number of observations.
B)A method of explaining observations that appears contradictory.
C)A broadly applicable generalization with virtually no exceptions.
D)A method for approaching problems that is used by all scientists.
E)A tentative model that describes the underlying cause of a physical behavior.
A)A prediction based on a limited number of observations.
B)A method of explaining observations that appears contradictory.
C)A broadly applicable generalization with virtually no exceptions.
D)A method for approaching problems that is used by all scientists.
E)A tentative model that describes the underlying cause of a physical behavior.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
17
When using the scientific method, before experiments are done a ___________ should be established.
A)hypothesis
B)law
C)theory
D)conclusion
A)hypothesis
B)law
C)theory
D)conclusion

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
18
Which scientist first theorized that matter was ultimately composed of small indivisible particles called atoms?
A)Dalton
B)Lavoisier
C)Empedocles
D)Plato
E)Democritus
A)Dalton
B)Lavoisier
C)Empedocles
D)Plato
E)Democritus

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
19
The scientific revolution of the 1500s was marked by a move away from ____ and towards ____ as a method for explaining the natural world.
A)law, theory
B)alchemy, research
C)reason, observation
D)scientific theory, experimentation
E)Both a and c are correct.
A)law, theory
B)alchemy, research
C)reason, observation
D)scientific theory, experimentation
E)Both a and c are correct.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
20
Which of these is not a requirement of a scientifically acceptable theory?
A)Good predictive power of the theory
B)Proven by additional experimentation
C)Sound reasoning for a particular observation
D)Easily revised to accommodate new observations
E)Provides model of behavior consistent with other widely accepted theories
A)Good predictive power of the theory
B)Proven by additional experimentation
C)Sound reasoning for a particular observation
D)Easily revised to accommodate new observations
E)Provides model of behavior consistent with other widely accepted theories

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
21
Which of these substances are heterogeneous mixtures?
I. Steam
II. Milk of magnesia
III. Crude oil
IV. Rubbing alcohol"
A)II and III
B)I and IV
C)II, III, and IV
D)I, III, and IV
E)I, II, and III
I. Steam
II. Milk of magnesia
III. Crude oil
IV. Rubbing alcohol"
A)II and III
B)I and IV
C)II, III, and IV
D)I, III, and IV
E)I, II, and III

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
22
If 12.0 g of carbon react with 32.0 g of oxygen to form 44.0 g of carbon dioxide, which of these statements is false?
A)18.0 g of carbon will be needed to form 66.0 g of carbon dioxide.
B)48.0 g of oxygen will be needed to form 66.0 g of carbon dioxide.
C)48.0 g of carbon will be needed to form 132.0 g of carbon dioxide.
D)96.0 g of oxygen will be needed to form 132.0 g of carbon dioxide.
E)None of the above are false.
A)18.0 g of carbon will be needed to form 66.0 g of carbon dioxide.
B)48.0 g of oxygen will be needed to form 66.0 g of carbon dioxide.
C)48.0 g of carbon will be needed to form 132.0 g of carbon dioxide.
D)96.0 g of oxygen will be needed to form 132.0 g of carbon dioxide.
E)None of the above are false.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
23
A bowl of chocolate chip ice cream is best described as
A)a pure substance.
B)a homogenous mixture.
C)a heterogeneous mixture.
D)a compound which is a pure substance.
E)a chemical reaction between chocolate and vanilla ice cream.
A)a pure substance.
B)a homogenous mixture.
C)a heterogeneous mixture.
D)a compound which is a pure substance.
E)a chemical reaction between chocolate and vanilla ice cream.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
24
Which of these statements is true?
A)Solids are compressible and have variable shape.
B)Solids are incompressible and have variable shape.
C)Solids are compressible and have a fixed shape.
D)Solids are incompressible and have a fixed shape.
A)Solids are compressible and have variable shape.
B)Solids are incompressible and have variable shape.
C)Solids are compressible and have a fixed shape.
D)Solids are incompressible and have a fixed shape.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is true of ice when it melts to form liquid water?
A)A physical change occurs.
B)A chemical change occurs.
C)Both physical and chemical changes occur.
D)Neither a physical nor chemical change occurs.
A)A physical change occurs.
B)A chemical change occurs.
C)Both physical and chemical changes occur.
D)Neither a physical nor chemical change occurs.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
26
The smallest unit of a chemical compound is
A)an atom.
B)a molecule.
C)a nucleus.
D)an alpha particle.
E)None of the above are true.
A)an atom.
B)a molecule.
C)a nucleus.
D)an alpha particle.
E)None of the above are true.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
27
Carbon and oxygen react to form carbon dioxide. What mass of carbon dioxide is produced when 12.0 g of carbon react with 32.0 g of oxygen?
A)44 g
B)38 g
C)28 g
D)20 g
E)2.67 g
A)44 g
B)38 g
C)28 g
D)20 g
E)2.67 g

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
28
Which of these statements is true?
A)The composition of both mixtures and pure substances is variable.
B)The composition of both mixtures and pure substances is fixed.
C)The composition of mixtures is variable and the composition of pure substances is fixed.
D)The composition of mixtures is fixed and the composition of pure substances is variable.
E)The composition of both mixtures and pure substances can be fixed or variable.
A)The composition of both mixtures and pure substances is variable.
B)The composition of both mixtures and pure substances is fixed.
C)The composition of mixtures is variable and the composition of pure substances is fixed.
D)The composition of mixtures is fixed and the composition of pure substances is variable.
E)The composition of both mixtures and pure substances can be fixed or variable.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
29
A substance composed of two or more different elements in fixed proportions is known as a(n)____.
A)atom
B)element
C)molecule
D)Ion
E)compound
A)atom
B)element
C)molecule
D)Ion
E)compound

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
30
A chocolate chip cookie is an example of what type of matter?
A)Element
B)Compound
C)Homogeneous mixture
D)Heterogeneous mixture
E)A cookie is not matter.
A)Element
B)Compound
C)Homogeneous mixture
D)Heterogeneous mixture
E)A cookie is not matter.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
31
Which of these substances are compounds?
I.Neon
II.Crude oil
III.Water
IV.Sodium chloride
A)I
B)I, and III
C)II, III, and IV
D)III and IV
E)II and IV
I.Neon
II.Crude oil
III.Water
IV.Sodium chloride
A)I
B)I, and III
C)II, III, and IV
D)III and IV
E)II and IV

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
32
The simplest form of substance is known as a(n)____.
A)element
B)ion
C)mixture
D)nucleus
E)homogeneous
A)element
B)ion
C)mixture
D)nucleus
E)homogeneous

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
33
Which of these substances are mixtures?
I. Steam
II. Crude oil
III. Salt water
IV. Gun powder
V. Oxygen
VI. Mercury"
A)II and III
B)I and III
C)II, III, and IV
D)I, III, and V
E)II, III, IV, and VI
I. Steam
II. Crude oil
III. Salt water
IV. Gun powder
V. Oxygen
VI. Mercury"
A)II and III
B)I and III
C)II, III, and IV
D)I, III, and V
E)II, III, IV, and VI

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
34
What does the figure represent?
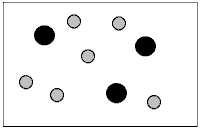
A)A heterogeneous mixture of elements.
B)A pure substance which is an element.
C)A pure substance which is a compound.
D)A homogenous mixture of elements and compounds.
E)A heterogeneous mixture of elements and compounds.

A)A heterogeneous mixture of elements.
B)A pure substance which is an element.
C)A pure substance which is a compound.
D)A homogenous mixture of elements and compounds.
E)A heterogeneous mixture of elements and compounds.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
35
Methane and oxygen react to form carbon dioxide and water. What mass of water is formed if 3.2 g of methane react with 12.8 g of oxygen to produce 8.8 g of carbon dioxide?
A)7.2 g
B)8.8 g
C)9.6 g
D)14.8 g
E)16.0 g
A)7.2 g
B)8.8 g
C)9.6 g
D)14.8 g
E)16.0 g

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
36
Methane can be decomposed into two simpler substances, hydrogen and carbon. Therefore, methane
A)is a gas.
B)is an element.
C)is a mixture.
D)is a compound.
E)must have the formula CH.
A)is a gas.
B)is an element.
C)is a mixture.
D)is a compound.
E)must have the formula CH.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is an example of a chemical change?
A)Glass breaking
B)Water freezing
C)Wood burning
D)Getting a haircut
A)Glass breaking
B)Water freezing
C)Wood burning
D)Getting a haircut

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following statements does not describe a chemical compound?.
A)It contains two or more elements.
B)It is a pure substance.
C)It has a fixed composition.
D)It has a variable composition.
E)All of the statements are correct.
A)It contains two or more elements.
B)It is a pure substance.
C)It has a fixed composition.
D)It has a variable composition.
E)All of the statements are correct.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
39
Which of these statements is true ?
A)Gases are compressible and have variable shape.
B)Gases are incompressible and have variable shape.
C)Gases are compressible and have a fixed shape.
D)Gases are incompressible and have a fixed shape.
A)Gases are compressible and have variable shape.
B)Gases are incompressible and have variable shape.
C)Gases are compressible and have a fixed shape.
D)Gases are incompressible and have a fixed shape.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
40
What does the figure represent?
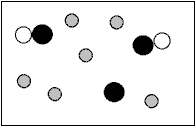
A)A heterogeneous mixture of elements.
B)A pure substance which is an element.
C)A pure substance which is a compound.
D)A homogenous mixture of elements and compounds.
E)A heterogeneous mixture of elements and compounds.

A)A heterogeneous mixture of elements.
B)A pure substance which is an element.
C)A pure substance which is a compound.
D)A homogenous mixture of elements and compounds.
E)A heterogeneous mixture of elements and compounds.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
41
A sample of heptane always contains 84% carbon and 16% hydrogen. Which of these best explains this phenomena?
A)Law of Constant Composition
B)Law of Conservation of Mass
C)Dalton's Atomic Theory
D)Law of Mass Action
E)Lavoisier's Law
A)Law of Constant Composition
B)Law of Conservation of Mass
C)Dalton's Atomic Theory
D)Law of Mass Action
E)Lavoisier's Law

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
42
Which scientist is responsible for establishing the Law of Conservation of Mass?
A)Bohr
B)Proust
C)Dalton
D)Lavoisier
E)Galileo
A)Bohr
B)Proust
C)Dalton
D)Lavoisier
E)Galileo

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
43
Analysis of a silicon dioxide (SiO2)sample indicated it contained 46.75 g of silicon and 53.25 g of oxygen. Determine the mass of silicon in a sample of SiO2 if the mass of oxygen is 21.3 g.
A)116.9 g
B)24.26
C)18.7 g
D)9.70 g
E)2.67 g
A)116.9 g
B)24.26
C)18.7 g
D)9.70 g
E)2.67 g

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is a characteristic of the nucleus of an atom?
A)It is positively charged.
B)It accounts for most of the mass of the atom.
C)It accounts for most of the volume of the atom.
D)Both B + C are correct.
E)Both A + B are correct.
A)It is positively charged.
B)It accounts for most of the mass of the atom.
C)It accounts for most of the volume of the atom.
D)Both B + C are correct.
E)Both A + B are correct.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
45
Which of these statements best explains the law of constant composition?
A)All atoms of a given element have the same weight.
B)Atoms of different elements combine in fixed whole number ratios.
C)The weight of an object is neither created nor destroyed in a chemical reaction.
D)All samples of a given compound have the same proportion of constituent elements.
E)The sum of the masses of the reactants equals the sum of the masses of the products in a normal chemical reaction.
A)All atoms of a given element have the same weight.
B)Atoms of different elements combine in fixed whole number ratios.
C)The weight of an object is neither created nor destroyed in a chemical reaction.
D)All samples of a given compound have the same proportion of constituent elements.
E)The sum of the masses of the reactants equals the sum of the masses of the products in a normal chemical reaction.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
46
Based on Rutherford's model of the atom, how many electrons would be found in an atom with 7 protons?
A)1
B)2
C)4
D)7
E)14
A)1
B)2
C)4
D)7
E)14

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
47
Elements A and Z react to form compound AZ. Compound AZ contains 40% A and60% Z by mass. Which statement best explains the outcome when 100 g of A is mixed with 100 g of Z?
A)The reaction will form 200 g of AZ.
B)The reaction will form 100 g of AZ.
C)After all possible AZ is formed, some Z will be left over.
D)After all possible AZ is formed, some A will be left over.
E)No reaction will occur since the reactants are in the wrong ratio.
A)The reaction will form 200 g of AZ.
B)The reaction will form 100 g of AZ.
C)After all possible AZ is formed, some Z will be left over.
D)After all possible AZ is formed, some A will be left over.
E)No reaction will occur since the reactants are in the wrong ratio.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
48
Which law best illustrates the following statement? Regardless of the amount of fluorine available, 23 g of sodium always combines with 19 g of fluorine.
A)Law of Constant Composition
B)Law of Conservation of Mass
C)Dalton's Atomic Theory
D)Law of Mass Action
E)Lavoisier's Law
A)Law of Constant Composition
B)Law of Conservation of Mass
C)Dalton's Atomic Theory
D)Law of Mass Action
E)Lavoisier's Law

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
49
Based on Rutherford's model of the atom, how many protons would be found in an atom with 17 electrons?
A)1
B)7
C)12
D)17
E)34
A)1
B)7
C)12
D)17
E)34

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
50
Which of these statements is not correct according to Dalton's Atomic Theory?
A)Elements combine in fixed proportions to form compounds.
B)Atoms are converted into other atoms in a chemical reaction.
C)All matter is composed of small indivisible particles called atoms.
D)Atoms of one element are different from atoms of another element.
E)All of these statements are correct according to Dalton's Atomic Theory.
A)Elements combine in fixed proportions to form compounds.
B)Atoms are converted into other atoms in a chemical reaction.
C)All matter is composed of small indivisible particles called atoms.
D)Atoms of one element are different from atoms of another element.
E)All of these statements are correct according to Dalton's Atomic Theory.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
51
Which of these statements is not consistent with Dalton's Atomic Theory?
A)All atoms of gold have the same chemical properties.
B)Electrons are equally distributed throughout an atom.
C)The properties of sodium are different from the properties of chlorine.
D)Compounds are formed when atoms combine in simple whole number ratios.
E)Atoms are rearranged in normal chemical reactions but are neither created nor destroyed.
A)All atoms of gold have the same chemical properties.
B)Electrons are equally distributed throughout an atom.
C)The properties of sodium are different from the properties of chlorine.
D)Compounds are formed when atoms combine in simple whole number ratios.
E)Atoms are rearranged in normal chemical reactions but are neither created nor destroyed.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
52
Analysis of a sodium chloride (NaCl)sample indicated that it contained 15 g of sodium and 23.1 g of chlorine. Determine the mass of chlorine in a sample of NaCl if the mass of sodium is 45.0 g.
A)29.2 g
B)60.0 g
C)68.1 g
D)69.3 g
E)83.1 g
A)29.2 g
B)60.0 g
C)68.1 g
D)69.3 g
E)83.1 g

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
53
Which scientist is responsible for establishing the concept of a nuclear atom?
A)Rutherford
B)Proust
C)Dalton
D)Lavoisier
E)Galileo
A)Rutherford
B)Proust
C)Dalton
D)Lavoisier
E)Galileo

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
54
10.00 g of the chemical compound benzene (C6H6)contains 0.77 g of hydrogen and 9.23 g of carbon. What mass of benzene will contain 10.00 g of hydrogen?
A)129.9 g
B)0.77 g
C)92.3 g
D)77.0 g
E)None of these.
A)129.9 g
B)0.77 g
C)92.3 g
D)77.0 g
E)None of these.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
55
Which of these sets of masses for nitrogen dioxide is not consistent with the others according to the Law of Constant Composition? 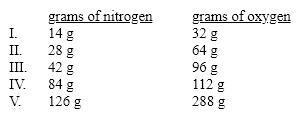
A)I
B)II
C)III
D)IV
E)V
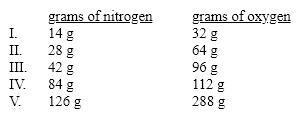
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
56
Which scientist is responsible for establishing the early atomic theory using the laws of Conservation of Mass and other related laws and observations?
A)Bohr
B)Proust
C)Dalton
D)Lavoisier
E)Galileo
A)Bohr
B)Proust
C)Dalton
D)Lavoisier
E)Galileo

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
57
Which of these statements best explains the Law of Conservation of Mass?
A)All atoms of a given element have the same weight.
B)Atoms of different elements combine in fixed whole number ratios.
C)The weight of an object is neither created nor destroyed in a chemical reaction.
D)All samples of a given compound have the same proportion of constituent elements.
E)The sum of the masses of the reactants equals the sum of the masses of the products in any normal chemical reaction.
A)All atoms of a given element have the same weight.
B)Atoms of different elements combine in fixed whole number ratios.
C)The weight of an object is neither created nor destroyed in a chemical reaction.
D)All samples of a given compound have the same proportion of constituent elements.
E)The sum of the masses of the reactants equals the sum of the masses of the products in any normal chemical reaction.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
58
Which of these statements is incorrect according to Rutherford's model of the atom?
A)Neutrons are part of the nucleus.
B)Most of the volume of an atom is empty space.
C)A neutral atom contains an equal number of protons and electrons.
D)An electron is located in close proximity to the nucleus of an atom.
E)The majority of the mass of the atom is concentrated in the nucleus.
A)Neutrons are part of the nucleus.
B)Most of the volume of an atom is empty space.
C)A neutral atom contains an equal number of protons and electrons.
D)An electron is located in close proximity to the nucleus of an atom.
E)The majority of the mass of the atom is concentrated in the nucleus.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
59
Which scientist is responsible for establishing the Law of Constant Composition?
A)Bohr
B)Proust
C)Dalton
D)Lavoisier
E)Galileo
A)Bohr
B)Proust
C)Dalton
D)Lavoisier
E)Galileo

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
60
2.0 g of hydrogen react with 16.0 g of oxygen to form 18.0 g of water. If 3.0 g of hydrogen is reacted with 16.0 g of oxygen, which of the following is true?
A)18.0 g of water will form with 1.0 g of excess oxygen.
B)18.0 g of water will form with 1.0 g of excess hydrogen.
C)19.0 g of water will form.
D)17.0 g of water will form with 1.0 g of excess oxygen and 1.0 g of excess hydrogen.
E)None of the above is correct.
A)18.0 g of water will form with 1.0 g of excess oxygen.
B)18.0 g of water will form with 1.0 g of excess hydrogen.
C)19.0 g of water will form.
D)17.0 g of water will form with 1.0 g of excess oxygen and 1.0 g of excess hydrogen.
E)None of the above is correct.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
61
Which scientist is incorrectly matched with his idea or theory? 
A)I.
B)II.
C)III.
D)IV.
E)V.

A)I.
B)II.
C)III.
D)IV.
E)V.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
62
Which two subatomic particles compose the nucleus of an atom?
A)Protons, neutrons
B)Protons, electrons
C)Electrons, neutrons
D)Alpha particles, protons
E)Alpha particles, electrons
A)Protons, neutrons
B)Protons, electrons
C)Electrons, neutrons
D)Alpha particles, protons
E)Alpha particles, electrons

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
63
Which scientist is incorrectly matched with his idea or theory?

A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
64
Which of these subatomic particles is not found in nucleus of an atom?
A)Proton
B)Neutron
C)Electron
D)Both A and B
E)None of these
A)Proton
B)Neutron
C)Electron
D)Both A and B
E)None of these

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
65
A college freshman reports to Student Health Services on campus complaining of a soar throat and fever. The doctor swabs the back of the student's throat and begins a throat culture. The swabbing and growing of a culture is an example of what step in the scientific method?
A)law
B)Theory
C)Conclusion
D)Observation
E)Experimentation
A)law
B)Theory
C)Conclusion
D)Observation
E)Experimentation

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
66
Which of these statements about science is incorrect ?
A)Science influences culture and society.
B)Science reveals knowledge not attainable by other means.
C)Science is a fundamental way to understand the world around us.
D)Decisions involving scientific principles are often made by nonscientists.
E)All of these are correct statements.
A)Science influences culture and society.
B)Science reveals knowledge not attainable by other means.
C)Science is a fundamental way to understand the world around us.
D)Decisions involving scientific principles are often made by nonscientists.
E)All of these are correct statements.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck


