Deck 3: Atoms and Elements
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/83
Play
Full screen (f)
Deck 3: Atoms and Elements
1
Determine the correct number of protons and electrons in F?.
A)9 protons, 10 electrons
B)9 protons, 11 electrons
C)10 protons, 9 electrons
D)11 protons, 10 electrons
E)19 protons, 20 electrons
A)9 protons, 10 electrons
B)9 protons, 11 electrons
C)10 protons, 9 electrons
D)11 protons, 10 electrons
E)19 protons, 20 electrons
9 protons, 10 electrons
2
What is the charge on an ion that contains 16 protons and 18 electrons?
A)0
B)+2
C)− 2
D)It depends on the number of neutrons.
E)None of these.
A)0
B)+2
C)− 2
D)It depends on the number of neutrons.
E)None of these.
− 2
3
Which of the following is not true for an atom that has an atomic number of 19 and a mass number of 41?
A)The atom contains 22 neutrons.
B)The atom is potassium.
C)The atom contains 19 neutrons.
D)The atom contains 19 electrons.
E)The atom contains 19 protons.
A)The atom contains 22 neutrons.
B)The atom is potassium.
C)The atom contains 19 neutrons.
D)The atom contains 19 electrons.
E)The atom contains 19 protons.
The atom contains 19 neutrons.
4
Which of these is the correct chemical symbol for cobalt?
A)C
B)Cl
C)Co
D)Ca
E)Ce
A)C
B)Cl
C)Co
D)Ca
E)Ce

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
5
Which of these is the correct chemical symbol for carbon?
A)C
B)Ca
C)Cl
D)Cb
E)Co
A)C
B)Ca
C)Cl
D)Cb
E)Co

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
6
Which of these particles has a negative charge?
A)Proton
B)Neutron
C)Electron
D)Nucleus
E)An atom
A)Proton
B)Neutron
C)Electron
D)Nucleus
E)An atom

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
7
When a strontium atom loses two electrons, it becomes a(n)_____ with a charge of _____.
A)anion, 2+
B)anion, 2 −
C)cation, 2 −
D)cation, 2+
E)cation, 0
A)anion, 2+
B)anion, 2 −
C)cation, 2 −
D)cation, 2+
E)cation, 0

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
8
A certain ion contains 47 protons and 46 electrons. The ion is:
A)Pd+
B)Pd −
C)Ag+
D)Ag −
E)Can't tell from the given information
A)Pd+
B)Pd −
C)Ag+
D)Ag −
E)Can't tell from the given information

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
9
Which of these statements about atoms is incorrect ?
A)Atoms are the smallest visible amount of an element.
B)Atoms are composed of protons, neutrons, and electrons.
C)Atoms are the building blocks of all matter.
D)Atoms are the smallest identifiable unit of an element.
E)There are 91 different naturally occurring atoms.
A)Atoms are the smallest visible amount of an element.
B)Atoms are composed of protons, neutrons, and electrons.
C)Atoms are the building blocks of all matter.
D)Atoms are the smallest identifiable unit of an element.
E)There are 91 different naturally occurring atoms.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
10
Nonmetals tend to _____ electrons to form ions of _____ charge.
A)lose, negative
B)lose, positive
C)gain, positive
D)gain, negative
A)lose, negative
B)lose, positive
C)gain, positive
D)gain, negative

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
11
Which of these is the correct chemical symbol for potassium?
A)K
B)Po
C)P
D)Pd
E)Pt
A)K
B)Po
C)P
D)Pd
E)Pt

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
12
When an oxygen atom gains two electrons, it becomes a(n)_____ with a charge of _____.
A)anion, 2+
B)anion, 2 −
C)cation, 2 −
D)cation, 2+
E)cation, 0
A)anion, 2+
B)anion, 2 −
C)cation, 2 −
D)cation, 2+
E)cation, 0

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
13
The most abundant element in the earth's crust by mass is:
A)Hydrogen
B)Carbon
C)Oxygen
D)Silicon
E)Nitrogen
A)Hydrogen
B)Carbon
C)Oxygen
D)Silicon
E)Nitrogen

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
14
The number of protons in a neutral atom is equal to the _____ of the atom.
A)mass
B)atomic weight
C)atomic number
D)mass number
E)valence electron number
A)mass
B)atomic weight
C)atomic number
D)mass number
E)valence electron number

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
15
Which statement about electrons is incorrect ?
A)Electrons are negatively charged.
B)Electrons have a negligible mass compared to the rest of the atom.
C)Electrons exist in the nucleus of the atom.
D)The number of electrons equals the atomic number in a neutral atom.
E)Both B and D are incorrect statements.
A)Electrons are negatively charged.
B)Electrons have a negligible mass compared to the rest of the atom.
C)Electrons exist in the nucleus of the atom.
D)The number of electrons equals the atomic number in a neutral atom.
E)Both B and D are incorrect statements.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
16
How many electrons are there in 15N3 − ?
A)15
B)18
C)12
D)4
E)10
A)15
B)18
C)12
D)4
E)10

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
17
Metals tend to _____ electrons to form ions of _____ charge.
A)lose, negative
B)lose, positive
C)gain, positive
D)gain, negative
A)lose, negative
B)lose, positive
C)gain, positive
D)gain, negative

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
18
Determine the correct number of protons and electrons in P3 − .
A)3 protons, 6 electrons
B)15 protons, 18 electrons
C)15 protons, 12 electrons
D)31 protons, 28 electrons
E)31 protons, 34 electrons
A)3 protons, 6 electrons
B)15 protons, 18 electrons
C)15 protons, 12 electrons
D)31 protons, 28 electrons
E)31 protons, 34 electrons

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
19
Which of these particles has the smallest mass?
A)A proton
B)A neutron
C)An electron
D)A hydrogen atom
E)A hydrogen nucleus
A)A proton
B)A neutron
C)An electron
D)A hydrogen atom
E)A hydrogen nucleus

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
20
Which of these statements about an atom is incorrect ?
A)The number protons equals the number of electrons.
B)The number of protons equals the atomic number.
C)The number of protons equals the number of neutrons.
D)The mass number is the sum of protons and neutrons in the atom.
E)The mass number minus the atomic number equals the number of neutrons in the atom.
A)The number protons equals the number of electrons.
B)The number of protons equals the atomic number.
C)The number of protons equals the number of neutrons.
D)The mass number is the sum of protons and neutrons in the atom.
E)The mass number minus the atomic number equals the number of neutrons in the atom.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
21
Carbon has three isotopes: 12C, 13C, and 14C. These isotopes each have _____ protons, _____ electrons and _____ , _____ , and _____ neutrons respectively.
A)6, 6, and 6, 7, 8
B)12, 12, and 6, 7, 8
C)12, 12, and 12, 13, 14
D)6, 6, and 12, 13, 14
E)12, 12, and 24, 25, 26
A)6, 6, and 6, 7, 8
B)12, 12, and 6, 7, 8
C)12, 12, and 12, 13, 14
D)6, 6, and 12, 13, 14
E)12, 12, and 24, 25, 26

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
22
Radon − 222 has _____ protons, _____ neutrons, and _____ electrons.
A)86, 86, 86
B)86, 222, 86
C)86, 136, 86
D)86, 136, 222
E)86, 222, 308
A)86, 86, 86
B)86, 222, 86
C)86, 136, 86
D)86, 136, 222
E)86, 222, 308

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
23
Which of these is the correct representation for the ion with Z=27, A=60, and C=2+ ?
A)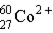
B)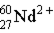
C)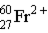
D)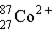
E)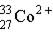
A)
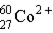
B)
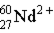
C)
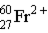
D)
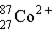
E)
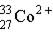

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
24
Which of these statements best describes the formation of an anion?
A)An atom loses one or more electrons and becomes positively charged.
B)An atom loses one or more electrons and becomes negatively charged.
C)An atom gains one or more electrons and becomes positively charged.
D)An atom gains one or more electrons and becomes negatively charged.
E)None of these are correct.
A)An atom loses one or more electrons and becomes positively charged.
B)An atom loses one or more electrons and becomes negatively charged.
C)An atom gains one or more electrons and becomes positively charged.
D)An atom gains one or more electrons and becomes negatively charged.
E)None of these are correct.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
25
An element has two naturally occurring isotopes. One has an abundance of 37.4% and an isotopic mass of 184.953 amu. The other has an abundance of 62.6% and a mass of 186.956 amu. What is the atomic weight of the element?
A)185.702 amu
B)185.954 amu
C)186.207 amu
D)186.956 amu
E)190.234 amu
A)185.702 amu
B)185.954 amu
C)186.207 amu
D)186.956 amu
E)190.234 amu

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
26
Isotopes of an element have the same number of _____ but a different number of _____.
A)neutrons, protons
B)electrons, protons
C)protons, neutrons
D)neutrons, electrons
E)protons, electrons
A)neutrons, protons
B)electrons, protons
C)protons, neutrons
D)neutrons, electrons
E)protons, electrons

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
27
Mendeleev organized the elements of the periodic table
A)by increasing atomic number and similar properties.
B)by increasing atomic weight and similar properties.
C)by increasing number of electrons.
D)by increasing number of isotopes.
E)alphabetically.
A)by increasing atomic number and similar properties.
B)by increasing atomic weight and similar properties.
C)by increasing number of electrons.
D)by increasing number of isotopes.
E)alphabetically.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
28
Rubidium consists of two naturally occurring isotopes rubidium-85 and rubidium-87. Rubidium-85 (85Rb)has an isotopic mass of 84.9117 amu and a 72.15% abundance. Rubidium-87(87Rb)has a 27.85% abundance. The atomic weight of rubidium is 85.4768 amu. Determine the isotopic mass of 87Rb?
A)86.72371 amu
B)86.8013 amu
C)86.8220 amu
D)86.8621 amu
E)86.9085 amu
A)86.72371 amu
B)86.8013 amu
C)86.8220 amu
D)86.8621 amu
E)86.9085 amu

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
29
What are the Z and A values for an atom of krypton which contains 46 neutrons?
A)Z=36, A=46
B)Z=46, A=36
C)Z=36, A=82
D)Z=82, A=36
E)Z=82, A=46
A)Z=36, A=46
B)Z=46, A=36
C)Z=36, A=82
D)Z=82, A=36
E)Z=82, A=46

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
30
The Ca2+ ion contains _____ protons and _____ electrons.
A)20, 20
B)20, 22
C)22, 20
D)18, 20
E)20, 18
A)20, 20
B)20, 22
C)22, 20
D)18, 20
E)20, 18

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
31
How many protons (p), neutrons (n), and electrons (e)are in an atom of 235U?
A)92 p, 146 n, 92 e
B)92 p, 143 n, 92 e
C)92 p, 92 n, 146 e
D)92 p, 92 n, 92 e
E)92 p, 51 n, 92 e
A)92 p, 146 n, 92 e
B)92 p, 143 n, 92 e
C)92 p, 92 n, 146 e
D)92 p, 92 n, 92 e
E)92 p, 51 n, 92 e

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
32
Copper has two naturally occurring isotopes. Cu-63 has a mass of 62.939 amu and Cu-65 has a mass of 64.928 amu. Based on the atomic mass of copper, which isotope has a higher fractional abundance?
A)Cu-63
B)Cu-65
C)Both have the same fractional abundance.
D)Not enough information to tell.
A)Cu-63
B)Cu-65
C)Both have the same fractional abundance.
D)Not enough information to tell.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
33
Which scientist is responsible for the organization of the modern periodic table?
A)Bohr
B)Galileo
C)Dalton
D)Avogadro
E)Mendeleev
A)Bohr
B)Galileo
C)Dalton
D)Avogadro
E)Mendeleev

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
34
Which of these statements best describes the formation of a cation?
A)An atom loses one or more electrons and becomes positively charged.
B)An atom loses one or more electrons and becomes negatively charged.
C)An atom gains one or more electrons and becomes positively charged.
D)An atom gains one or more electrons and becomes negatively charged.
E)None of these are correct.
A)An atom loses one or more electrons and becomes positively charged.
B)An atom loses one or more electrons and becomes negatively charged.
C)An atom gains one or more electrons and becomes positively charged.
D)An atom gains one or more electrons and becomes negatively charged.
E)None of these are correct.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
35
Metals tend to form ions with a positive charge. The most likely way to form a positively charged ion from an atom is by doing which of the following?
A)Gaining electron(s).
B)Gaining proton(s).
C)Losing electron(s).
D)Losing proton(s).
E)Losing neutron(s).
A)Gaining electron(s).
B)Gaining proton(s).
C)Losing electron(s).
D)Losing proton(s).
E)Losing neutron(s).

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
36
How many protons, neutrons, and electrons are in an 25Mg+2 ion?.
A)12 protons, 12 electrons, 13 neutrons
B)12 protons, 10 electrons, 12 neutrons
C)13 protons, 10 electrons, 12 neutrons
D)12 protons, 10 electrons, 13 neutrons
E)12 protons, 14 electrons, 13 neutrons
A)12 protons, 12 electrons, 13 neutrons
B)12 protons, 10 electrons, 12 neutrons
C)13 protons, 10 electrons, 12 neutrons
D)12 protons, 10 electrons, 13 neutrons
E)12 protons, 14 electrons, 13 neutrons

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
37
Chlorine has two isotopes, 35Cl and 37Cl that have the respective percent abundance's of 75.77% and 24.23% and masses of 34.9688 amu and 36.9659 amu. Calculate the atomic weight of chlorine.
A)37.54 amu
B)36.65 amu
C)35.45 amu
D)34.97 amu
E)36.97 amu
A)37.54 amu
B)36.65 amu
C)35.45 amu
D)34.97 amu
E)36.97 amu

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
38
The Cl − ion contains _____ protons and _____ electrons.
A)17, 17
B)17, 18
C)18, 16
D)18, 18
E)16, 17
A)17, 17
B)17, 18
C)18, 16
D)18, 18
E)16, 17

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
39
What are the Z and A values for an atom of silicon which contains 15 neutrons?
A)Z=14, A=15
B)Z=15, A=14
C)Z=29, A=14
D)Z=14, A=29
E)Z=15, A=29
A)Z=14, A=15
B)Z=15, A=14
C)Z=29, A=14
D)Z=14, A=29
E)Z=15, A=29

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
40
There are three isotopes of neon. Assuming the mass of each isotope is very close to its mass number, which isotope is most abundant based on the atomic mass of neon (Ne)?
A)Ne-20
B)Ne-21
C)Ne-22
D)Can't tell from the given information.
A)Ne-20
B)Ne-21
C)Ne-22
D)Can't tell from the given information.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
41
Elements in the same group have the same _____.
A)mass number
B)atomic number
C)number of protons
D)number of electrons
E)number of valence electrons
A)mass number
B)atomic number
C)number of protons
D)number of electrons
E)number of valence electrons

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
42
Which of these elements is a metal?
A)Mg
B)Br
C)Ar
D)Ge
E)B
A)Mg
B)Br
C)Ar
D)Ge
E)B

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
43
Which element is not a nonmetal?
A)P
B)I
C)N
D)K
E)Cl
A)P
B)I
C)N
D)K
E)Cl

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
44
The elements of group 7A will all form ions. These ions will be _____ and will have a charge of _____.
A)cations, 2+
B)anions, 2 −
C)cations, 3+
D)anions, 1 −
E)cations, 1+
A)cations, 2+
B)anions, 2 −
C)cations, 3+
D)anions, 1 −
E)cations, 1+

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
45
Which of these elements is a metalloid?
A)Ge
B)Br
C)Ar
D)Ca
E)Fe
A)Ge
B)Br
C)Ar
D)Ca
E)Fe

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
46
What is the maximum number of electrons which can occupy the orbit n =2?
A)2
B)6
C)8
D)10
E)18
A)2
B)6
C)8
D)10
E)18

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
47
Which of these elements has seven valence electrons?
A)Mg
B)Li
C)Al
D)S
E)Cl
A)Mg
B)Li
C)Al
D)S
E)Cl

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
48
Which scientist is responsible for the theory which explains why properties of elements recur in a periodic fashion?
A)Bohr
B)Galileo
C)Dalton
D)Avogadro
E)Mendeleev
A)Bohr
B)Galileo
C)Dalton
D)Avogadro
E)Mendeleev

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
49
Which of these elements has three valence electrons?
A)Mg
B)Li
C)Al
D)S
E)Cl
A)Mg
B)Li
C)Al
D)S
E)Cl

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following elements is generally not found in diatomic form in nature?
A)C
B)N
C)O
D)F
A)C
B)N
C)O
D)F

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is true?
A)Argon and chlorine have the same number of electrons.
B)Argon and chlorine have the same number of valence electrons.
C)Argon and neon have the same number of electrons.
D)Argon and neon have the same number of valence electrons.
E)There is more than one correct statement.
A)Argon and chlorine have the same number of electrons.
B)Argon and chlorine have the same number of valence electrons.
C)Argon and neon have the same number of electrons.
D)Argon and neon have the same number of valence electrons.
E)There is more than one correct statement.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
52
The elements of group IA will all form ions. These ions will be _____ and will have a charge of _____.
A)cations, 2+
B)anions, 2 −
C)cations, 3+
D)anions, 1 −
E)cations, 1+
A)cations, 2+
B)anions, 2 −
C)cations, 3+
D)anions, 1 −
E)cations, 1+

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
53
Which of these elements has two valence electrons?
A)Mg
B)Li
C)Al
D)S
E)Cl
A)Mg
B)Li
C)Al
D)S
E)Cl

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is true?
A)Argon and chlorine have a different number of electrons.
B)Argon and chlorine have a different number of valence electrons.
C)Argon and neon have a different number of electrons.
D)Argon and neon have a different number of valence electrons.
E)There is more than one correct statement.
A)Argon and chlorine have a different number of electrons.
B)Argon and chlorine have a different number of valence electrons.
C)Argon and neon have a different number of electrons.
D)Argon and neon have a different number of valence electrons.
E)There is more than one correct statement.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
55
He has the same number of valence electrons as _____.
A)H
B)Ne
C)Be
D)F
A)H
B)Ne
C)Be
D)F

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
56
Which of these statements about Bohr's model of the atom is incorrect ?
A)Electrons fill lower energy orbits first.
B)The greater the quantum number the higher the energy of an orbit.
C)Electrons occupy orbits with certain radii corresponding to discrete energies.
D)Elements with the same number of electrons in their outer orbit have similar properties.
E)All of these statements are correct.
A)Electrons fill lower energy orbits first.
B)The greater the quantum number the higher the energy of an orbit.
C)Electrons occupy orbits with certain radii corresponding to discrete energies.
D)Elements with the same number of electrons in their outer orbit have similar properties.
E)All of these statements are correct.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
57
These elements Li, Na, K, Rb, and Cs are members of what group\family?
A)halogens
B)noble gases
C)alkali metals
D)transition metals
E)alkaline earth metals
A)halogens
B)noble gases
C)alkali metals
D)transition metals
E)alkaline earth metals

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
58
Which of these statements concerning atomic mass is correct?
A)Atomic mass is the same as the mass number of the most common isotope.
B)Atomic mass is derived from the weight of the most common isotope of the element.
C)Atomic mass is determined by adding the masses of the protons, electrons, and neutrons in the atom.
D)Atomic mass is calculated from the atomic mass of the two most common isotopes.
E)Atomic mass is a weighted average of the masses of the naturally occurring isotopes.
A)Atomic mass is the same as the mass number of the most common isotope.
B)Atomic mass is derived from the weight of the most common isotope of the element.
C)Atomic mass is determined by adding the masses of the protons, electrons, and neutrons in the atom.
D)Atomic mass is calculated from the atomic mass of the two most common isotopes.
E)Atomic mass is a weighted average of the masses of the naturally occurring isotopes.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
59
What is the total number of electrons which can occupy the first and second principal energy levels ( n =1, n =2)?
A)2
B)6
C)8
D)10
E)18
A)2
B)6
C)8
D)10
E)18

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
60
Which of these is a nonmetal?
A)Mg
B)Br
C)Cu
D)Ge
E)Li
A)Mg
B)Br
C)Cu
D)Ge
E)Li

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
61
One mole of nickel (Ni)has a mass of _____.
A)28 amu
B)28 g
C)58.71 amu
D)58.71 g
A)28 amu
B)28 g
C)58.71 amu
D)58.71 g

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
62
What is the correct number of atoms in one mole of aluminum?
A)12
B)26.98
C)6.022 × 1023
D)13
E)14
A)12
B)26.98
C)6.022 × 1023
D)13
E)14

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
63
Calculate the number of moles in a 25.0 g sample of carbon.
A)2.08 moles
B)13.0 moles
C)300 moles
D)1.51 × 1025 moles
E)3.33 × 10 − 3 moles
A)2.08 moles
B)13.0 moles
C)300 moles
D)1.51 × 1025 moles
E)3.33 × 10 − 3 moles

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
64
Calculate the number of moles in a 2.98 g sample of aluminum.
A)0.0124 moles
B)0.110 moles
C)80.4 moles
D)1.79 × 1024 moles
E)1.62 × 1025 moles
A)0.0124 moles
B)0.110 moles
C)80.4 moles
D)1.79 × 1024 moles
E)1.62 × 1025 moles

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
65
Determine the mass in grams of one molecule of water, H2O.
A)2.99 × 10 − 23 g
B)1.08 × 1025 g
C)10 grams
D)18 grams
E)None of the above.
A)2.99 × 10 − 23 g
B)1.08 × 1025 g
C)10 grams
D)18 grams
E)None of the above.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following is true?
A)Oxygen and sulfur are in the same group.
B)Oxygen and sulfur are in different groups.
C)Oxygen and sulfur are in the same period.
D)Oxygen and sulfur are in different periods.
E)Both A and D are correct.
A)Oxygen and sulfur are in the same group.
B)Oxygen and sulfur are in different groups.
C)Oxygen and sulfur are in the same period.
D)Oxygen and sulfur are in different periods.
E)Both A and D are correct.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
67
What is the correct number of atoms in one mole of neon?
A)10
B)11
C)6.022 × 1023
D)20
E)12
A)10
B)11
C)6.022 × 1023
D)20
E)12

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
68
Determine the number of atoms in a copper strip that has a mass of 35.0 grams.
A)3.33 × 1025 atoms
B)2.11 × 1025 atoms
C)2.71 × 1020 atoms
D)3.32 × 1023 atoms
E)0.551 atoms
A)3.33 × 1025 atoms
B)2.11 × 1025 atoms
C)2.71 × 1020 atoms
D)3.32 × 1023 atoms
E)0.551 atoms

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
69
Which family\group is largely unreactive?
A)Halogens
B)Noble gases
C)Alkali metals
D)Transition metals
E)Alkaline earth metals
A)Halogens
B)Noble gases
C)Alkali metals
D)Transition metals
E)Alkaline earth metals

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
70
Determine the mass of one mole of silver.
A)107.87 amu
B)107.87 g
C)47 amu
D)47 g
E)6.022 × 1023 amu
A)107.87 amu
B)107.87 g
C)47 amu
D)47 g
E)6.022 × 1023 amu

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
71
Determine the number of atoms in a piece of ultrapure silicon that has a mass of 15.0 grams.
A)1.13 × 1024 atoms
B)1.69 × 1025 atoms
C)2.54 × 1020 atoms
D)3.21 × 1023 atoms
E)9.03 × 1024 atoms
A)1.13 × 1024 atoms
B)1.69 × 1025 atoms
C)2.54 × 1020 atoms
D)3.21 × 1023 atoms
E)9.03 × 1024 atoms

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
72
Bromine is a member of what group\family?
A)halogens
B)noble gases
C)alkali metals
D)transition metals
E)alkaline earth metals
A)halogens
B)noble gases
C)alkali metals
D)transition metals
E)alkaline earth metals

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
73
The atomic mass of fluorine (F)is 19.00. Which of the following is not true?
A)An atom of F has a mass of 19.00 amu.
B)One mole of F has a mass of 19.00 g.
C)6.02 x 1023 atoms of F has a mass of 19.00 g.
D)6.02 x 1023 atoms of F has a mass of 19.00 amu.
A)An atom of F has a mass of 19.00 amu.
B)One mole of F has a mass of 19.00 g.
C)6.02 x 1023 atoms of F has a mass of 19.00 g.
D)6.02 x 1023 atoms of F has a mass of 19.00 amu.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
74
Determine the mass in grams of 1.15 × 1023 atoms of cobalt.
A)0.191 grams
B)5.23 grams
C)11.2 grams
D)12.4 grams
E)308 grams
A)0.191 grams
B)5.23 grams
C)11.2 grams
D)12.4 grams
E)308 grams

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
75
Which of these elements is not a transition metal?
A)Cu
B)Fe
C)Na
D)Ag
E)Hg
A)Cu
B)Fe
C)Na
D)Ag
E)Hg

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
76
Determine the mass in grams of 3.45 × 1025 molecules of oxygen, O2.
A)0.556 grams
B)57.2 grams
C)458 grams
D)916 grams
E)1.83 × 103 grams
A)0.556 grams
B)57.2 grams
C)458 grams
D)916 grams
E)1.83 × 103 grams

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
77
Which of these groups is incorrectly matched with its group\family name?

A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
78
Calculate the number of moles in a 233 gram sample of barium.
A)0.589 moles
B)1.70 moles
C)3.20 × 104
D)6.022 × 1023 moles
E)1.02 × 1024 moles
A)0.589 moles
B)1.70 moles
C)3.20 × 104
D)6.022 × 1023 moles
E)1.02 × 1024 moles

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
79
Strontium is a member of what group\family?
A)halogens
B)noble gases
C)alkali metals
D)transition metals
E)alkaline earth metals
A)halogens
B)noble gases
C)alkali metals
D)transition metals
E)alkaline earth metals

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
80
Calculate the number of moles of lead in a 442 gram lead anvil.
A)0.468 moles
B)2.13 moles
C)96.97 moles
D)6.022 × 1023 moles
E)1.28 × 1024 moles
A)0.468 moles
B)2.13 moles
C)96.97 moles
D)6.022 × 1023 moles
E)1.28 × 1024 moles

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck


