Deck 11: The Air Around Us
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/67
Play
Full screen (f)
Deck 11: The Air Around Us
1
What causes pressure?
A)Constant small fluctuations in temperature
B)Collisions of gas molecules with other gas molecules
C)Collisions of gas molecules with their container
D)Weather
E)None of the above
A)Constant small fluctuations in temperature
B)Collisions of gas molecules with other gas molecules
C)Collisions of gas molecules with their container
D)Weather
E)None of the above
Collisions of gas molecules with their container
2
The layer of air which surrounds the earth is known as the ____.
A)crust
B)pressure
C)atmosphere
D)gas constant
E)Boyle's constant
A)crust
B)pressure
C)atmosphere
D)gas constant
E)Boyle's constant
atmosphere
3
Pressure is defined as:
A)force times unit area.
B)mass times acceleration.
C)mass divided by density.
D)force divided by unit area.
E)mass divided by acceleration.
A)force times unit area.
B)mass times acceleration.
C)mass divided by density.
D)force divided by unit area.
E)mass divided by acceleration.
force divided by unit area.
4
Which of these is the correct representation of Boyle's law?
A)PV = k
B)V\T = k
C)P\V = k
D)V\P = k
E)VT = k
A)PV = k
B)V\T = k
C)P\V = k
D)V\P = k
E)VT = k

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
5
In a sample of a nearly ideal gas, this graph could represent a plot of
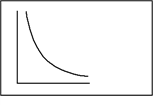
A)Boyle's law, V vs. T at a given constant P.
B)Charles's law, P vs. T at a given constant V.
C)Boyle's law, P vs. V at a given constant T.
D)Dalton's law, PV vs. P at a given constant T.
E)Avogadro's law, P vs. n at a constant T.

A)Boyle's law, V vs. T at a given constant P.
B)Charles's law, P vs. T at a given constant V.
C)Boyle's law, P vs. V at a given constant T.
D)Dalton's law, PV vs. P at a given constant T.
E)Avogadro's law, P vs. n at a constant T.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
6
Which of these is not a possible unit for pressure?
A)in. Hg
B)psi
C)torr
D)mm Hg
E)lb\s
A)in. Hg
B)psi
C)torr
D)mm Hg
E)lb\s

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
7
Which of these is not a possible unit for pressure?
A)mm Hg
B)psi
C)torr
D)atm
E)J\s
A)mm Hg
B)psi
C)torr
D)atm
E)J\s

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
8
According to Boyle's law, if the pressure of a gas is increased by a factor of four then the ____ of a gas is ____ by a factor of ____.
A)volume, increased, four
B)volume, decreased, four
C)volume, increased, fourteen
D)temperature, increased, four
E)temperature, decreased, four
A)volume, increased, four
B)volume, decreased, four
C)volume, increased, fourteen
D)temperature, increased, four
E)temperature, decreased, four

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
9
A balloon contains 14.0 L of air at a pressure of 760 torr. What will be the volume of the air when the balloon is taken to a region with a pressure of 981 torr at the same temperature ?
A)1.3 L
B)8.2 L
C)10.8 L
D)14.0 L
E)18 L
A)1.3 L
B)8.2 L
C)10.8 L
D)14.0 L
E)18 L

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
10
What is the correct conversion factor between atmospheres and torr?
A)1 atm = 1 torr
B)1 atm = 760 torr
C)760 atm = 1 torr
D)1 atm = 29.92 torr
A)1 atm = 1 torr
B)1 atm = 760 torr
C)760 atm = 1 torr
D)1 atm = 29.92 torr

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
11
Which of these is the correct representation of Charles's Law?
A)PV = k
B)V\T = k
C)P\V = k
D)V\P = k
E)VT = k
A)PV = k
B)V\T = k
C)P\V = k
D)V\P = k
E)VT = k

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
12
A sample of Freon gas in an air conditioner has a volume of 325 L and pressure of 96,300 Pa at 20 ° C. What will the pressure the Freon be when the volume is 975 L at 20 ° C?
A)1975 Pa
B)3.2 × 104 Pa
C)2.89 × 105 Pa
D)3.1 × 107 Pa
E)9.4 × 107 Pa
A)1975 Pa
B)3.2 × 104 Pa
C)2.89 × 105 Pa
D)3.1 × 107 Pa
E)9.4 × 107 Pa

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
13
A sample of acetylene gas occupies a volume of 47.2 L under a pressure of 1.63 atm at 25 ° C. What volume will the acetylene occupy if the pressure is decreased to 0.961 atm at 25 ° C?
A)27.8 L
B)30.0 L
C)32.3 L
D)47.8 L
E)80.1 L
A)27.8 L
B)30.0 L
C)32.3 L
D)47.8 L
E)80.1 L

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
14
Which of these statements about pressure is \are incorrect ?
I. Pressure is directly proportional to the number of gas molecules.
II. Pressure is directly proportional to temperature.
III. Pressure is directly proportional to volume.
A)I only
B)III only
C)I and II
D)I and III
E)II and III
I. Pressure is directly proportional to the number of gas molecules.
II. Pressure is directly proportional to temperature.
III. Pressure is directly proportional to volume.
A)I only
B)III only
C)I and II
D)I and III
E)II and III

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is the formula for the chemical compound found in airbags?
A)Na3N
B)NaN3
C)Na2O
D)NaO2
A)Na3N
B)NaN3
C)Na2O
D)NaO2

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
16
Which of these statements concerning pressure and weather are correct ?
I. Low pressure in a region tends to draw in storms.
II. High pressure in a region usually indicates clear weather.
III. Changes in pressure from region to region are responsible for winds.
A)I only
B)III only
C)I and II
D)I and III
E)I, II, and III
I. Low pressure in a region tends to draw in storms.
II. High pressure in a region usually indicates clear weather.
III. Changes in pressure from region to region are responsible for winds.
A)I only
B)III only
C)I and II
D)I and III
E)I, II, and III

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
17
A gas contained in a piston has a pressure of 100. torr and a volume of 1.0 L. Determine the pressure of the gas if it is compressed to a volume of 0.75 L at the same temperature .
A)25 torr
B)55 torr
C)75 torr
D)133 torr
E)150 torr
A)25 torr
B)55 torr
C)75 torr
D)133 torr
E)150 torr

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
18
Which of these is not a possible unit for pressure?
A)g\mL
B)lb\in2
C)Pa
D)N\m2
E)mm Hg
A)g\mL
B)lb\in2
C)Pa
D)N\m2
E)mm Hg

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
19
Which of these laws describes the relationship between pressure and volume at a constant temperature?
A)Ideal Gas law
B)Boyle's law
C)Charles's law
D)Avogadro's law
E)Graham's law
A)Ideal Gas law
B)Boyle's law
C)Charles's law
D)Avogadro's law
E)Graham's law

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
20
According to Boyle's law, if the pressure of a gas is decreased by a factor of five then the ____ of a gas is ____ by a factor of ____.
A)volume, increased, five
B)volume, decreased, 1\5th
C)volume, increased, 15
D)temperature, increased, five
E)temperature, decreased, 1\5th
A)volume, increased, five
B)volume, decreased, 1\5th
C)volume, increased, 15
D)temperature, increased, five
E)temperature, decreased, 1\5th

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
21
The temperature scale used in gas law problems_________.
A)must be Celsius
B)must be Kelvin
C)must be Fahrenheit
D)does not matter
A)must be Celsius
B)must be Kelvin
C)must be Fahrenheit
D)does not matter

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
22
Which of these gases is the most abundant in the Earth's atmosphere?
A)O2
B)N2
C)H2
D)He
E)CO2
A)O2
B)N2
C)H2
D)He
E)CO2

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
23
Which of these is responsible for the rusting of metal and the dulling of paint?
A)N2
B)O2
C)Ar
D)He
E)CO2
A)N2
B)O2
C)Ar
D)He
E)CO2

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
24
The noble gas argon accounts for approximately what % of the earth's atmosphere?
A)10%
B)1%
C)0.1%
D)0.01%
E)0.001%
A)10%
B)1%
C)0.1%
D)0.01%
E)0.001%

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
25
Rank these gases in order of their abundance in the atmosphere (least to greatest).
(O2, N2, Ar, Ne, CO2)
A)O2 < N2 < Ar < Ne < CO2
B)N2 < O2 < Ar < Ne < CO2
C)Ar < Ne < CO2 < O2 < N2
D)Ne < CO2 < Ar < O2 < N2
E)CO2 < Ne < Ar < O2 < N2
(O2, N2, Ar, Ne, CO2)
A)O2 < N2 < Ar < Ne < CO2
B)N2 < O2 < Ar < Ne < CO2
C)Ar < Ne < CO2 < O2 < N2
D)Ne < CO2 < Ar < O2 < N2
E)CO2 < Ne < Ar < O2 < N2

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
26
According to Charles's law, if the volume of a gas is increased by a factor of ten then the ____ of a gas is ____ by a factor of ____.
A)volume, increased, ten
B)volume, decreased, ten
C)volume, increased, 1\10th
D)temperature, increased, ten
E)temperature, decreased, ten
A)volume, increased, ten
B)volume, decreased, ten
C)volume, increased, 1\10th
D)temperature, increased, ten
E)temperature, decreased, ten

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
27
A sample of gas occupies 30.0 L at 0 ° C and 1 atm. What temperature will the same gas have in a 22.5 L container with a pressure of 3.5 atm?
A)212 ° C
B)284 ° C
C)386 ° C
D)444 ° C
E)516 ° C
A)212 ° C
B)284 ° C
C)386 ° C
D)444 ° C
E)516 ° C

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
28
A sample of carbon dioxide at 10.0 ° C has a volume of 300. mL at 760. torr. What is the volume of the gas at 30.0 ° C and 760. torr?
A)275 mL
B)280 mL
C)321 mL
D)755 mL
E)900 mL
A)275 mL
B)280 mL
C)321 mL
D)755 mL
E)900 mL

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
29
The volume of 350. mL of gas at 25.0 ° C is decreased to 125 mL at constant pressure. What is the final temperature of the gas?
A)− 167 ° C
B)− 126 ° C
C)70 ° C
D)89 ° C
E)147 ° C
A)− 167 ° C
B)− 126 ° C
C)70 ° C
D)89 ° C
E)147 ° C

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
30
What is the approximate composition of the earth's atmosphere?
A)80 % oxygen and 20 % nitrogen
B)80 % nitrogen and 20 % oxygen
C)50 % nitrogen and 50 % oxygen
D)100 % oxygen
E)100 % nitrogen
A)80 % oxygen and 20 % nitrogen
B)80 % nitrogen and 20 % oxygen
C)50 % nitrogen and 50 % oxygen
D)100 % oxygen
E)100 % nitrogen

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
31
If a gas sample is doubled in pressure and doubled in temperature, the volume will be:
A)a fourth of the original volume.
B)half of the original volume.
C)the same as the original volume.
D)twice the original volume.
E)four times the original volume.
A)a fourth of the original volume.
B)half of the original volume.
C)the same as the original volume.
D)twice the original volume.
E)four times the original volume.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
32
A sample of nitrogen at pressure P is contained in a sealed syringe with a movable piston. If the volume of the sample were doubled and the absolute temperature tripled, the new pressure of the gas would be:
A)6.0 P
B)5.0 P
C)3.5 P
D)1.5 P
E)0.5 P
A)6.0 P
B)5.0 P
C)3.5 P
D)1.5 P
E)0.5 P

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
33
According to Charles's law, if the volume of a gas is decreased by a factor of three then the ____ of a gas is ____ by a factor of ____.
A)volume, increased, three
B)volume, decreased, 1\3th
C)volume, increased, 9
D)temperature, increased, three
E)temperature, decreased, three
A)volume, increased, three
B)volume, decreased, 1\3th
C)volume, increased, 9
D)temperature, increased, three
E)temperature, decreased, three

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
34
A basketball is inflated to a pressure of 1.50 atm in a 20.0 ° C garage. What is the pressure of the basketball outside where the temperature is − 5.00 ° C?
A)0.375 atm
B)1.37 atm
C)1.42 atm
D)1.67 atm
E)3.01 atm
A)0.375 atm
B)1.37 atm
C)1.42 atm
D)1.67 atm
E)3.01 atm

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
35
Combining Charles's law and Boyle's law results in which equation?
A)PVT = k
B)PT\V = k
C)P\VT = k
D)PV\T = k
E)None of the above
A)PVT = k
B)PT\V = k
C)P\VT = k
D)PV\T = k
E)None of the above

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
36
Rank these gases in order of their abundance in the atmosphere (least to greatest).
(O2, N2, H2, He, CO2)
A)O2 < N2 < H2 < He < CO2
B)N2 < O2 < H2 < He < CO2
C)H2 < He < CO2 < O2 < N2
D)H2 < He < O2 < CO2 < N2
E)CO2 < He < H2 < O2 < N2
(O2, N2, H2, He, CO2)
A)O2 < N2 < H2 < He < CO2
B)N2 < O2 < H2 < He < CO2
C)H2 < He < CO2 < O2 < N2
D)H2 < He < O2 < CO2 < N2
E)CO2 < He < H2 < O2 < N2

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
37
Which of these gases is \are not a part (either as a reactant or as a product)of the biochemical reaction known as respiration?
I. N2
II. O2
III. H2O
IV. CO2
A)I only
B)I and III
C)II and III
D)I and IV
E)I, III, and IV
I. N2
II. O2
III. H2O
IV. CO2
A)I only
B)I and III
C)II and III
D)I and IV
E)I, III, and IV

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
38
Which of these gases is broken down by bacteria in the soil and used directly by plants?
A)N2
B)O2
C)Ar
D)He
E)CO2
A)N2
B)O2
C)Ar
D)He
E)CO2

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
39
A sample of oxygen with a volume of V is contained in a sealed syringe with a movable piston. If the pressure of the sample were halved and the absolute temperature tripled, what would be the new volume of the gas?
A)6.0 V
B)5.0 V
C)3.5 V
D)1.5 V
E)0.5 V
A)6.0 V
B)5.0 V
C)3.5 V
D)1.5 V
E)0.5 V

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
40
A sample of helium occupies 1.40 L at 0 ° C and 760 torr. What pressure will the same gas exert in a 0.500 L vessel at 100 ° C?
A)371 torr
B)624 torr
C)1.56 × 103 torr
D)2.91 × 103 torr
E)4.05 × 103 torr
A)371 torr
B)624 torr
C)1.56 × 103 torr
D)2.91 × 103 torr
E)4.05 × 103 torr

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
41
Which of these gases is absorbed by the earth's oceans, later forming rocks and sedimentation?
A)N2
B)O2
C)Ar
D)He
E)CO2
A)N2
B)O2
C)Ar
D)He
E)CO2

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
42
Which region of the Earth's atmosphere supports all earth-bound life?
A)Firstosphere
B)Mesosphere
C)Stratosphere
D)Troposphere
E)Ionosphere
A)Firstosphere
B)Mesosphere
C)Stratosphere
D)Troposphere
E)Ionosphere

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
43
Meteors burn up in this region of the atmosphere.
A)Firstosphere
B)Mesosphere
C)Stratosphere
D)Troposphere
E)Ionosphere
A)Firstosphere
B)Mesosphere
C)Stratosphere
D)Troposphere
E)Ionosphere

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
44
Which of these pollutants diminishes the bloods ability to carry oxygen?
A)CO
B)PM-2.5
C)SO2
D)O3
E)Pb
A)CO
B)PM-2.5
C)SO2
D)O3
E)Pb

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
45
Which statement is correct?
A)UV-A is more harmful to humans than UV-B.
B)UV-B is more harmful to humans than UV-A.
C)UV-A and UV-B are equally harmful to humans.
D)Neither UV-A or UV-B is harmful to humans.
A)UV-A is more harmful to humans than UV-B.
B)UV-B is more harmful to humans than UV-A.
C)UV-A and UV-B are equally harmful to humans.
D)Neither UV-A or UV-B is harmful to humans.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
46
Which of these pollutants gives smog its characteristic brown color?
A)CO
B)NO2
C)SO2
D)O3
E)Pb
A)CO
B)NO2
C)SO2
D)O3
E)Pb

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
47
Which of these gases is\ are considered inert and unreactive?
I. Ne
II. O2
III. Ar
IV. CO2
A)I only
B)II only
C)II and III
D)I and III
E)I, III, and IV
I. Ne
II. O2
III. Ar
IV. CO2
A)I only
B)II only
C)II and III
D)I and III
E)I, III, and IV

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
48
Which region of the atmosphere is credited with the phenomena known as aurora borealis or the northern lights?
A)Firstosphere
B)Mesosphere
C)Stratosphere
D)Troposphere
E)Ionosphere
A)Firstosphere
B)Mesosphere
C)Stratosphere
D)Troposphere
E)Ionosphere

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
49
Which of these pollutants can contribute to damage to the kidneys, liver, and nervous system?
A)CO
B)NO2
C)SO2
D)O3
E)Pb
A)CO
B)NO2
C)SO2
D)O3
E)Pb

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
50
Which of these acts as a pollutant in the troposphere, but as a UV shield in the stratosphere?
A)CO
B)PM-2.5
C)SO2
D)O3
E)Pb
A)CO
B)PM-2.5
C)SO2
D)O3
E)Pb

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
51
Nitrogen fixation converts nitrogen to ________.
A)cellulose
B)nitrates
C)nitrogen oxides
D)nitric acid
A)cellulose
B)nitrates
C)nitrogen oxides
D)nitric acid

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
52
According to data obtained by NAAQS, which of these pollutants is the most prominent pollutant problem in the United States?
A)CO
B)NO2
C)SO2
D)O3
E)Pb
A)CO
B)NO2
C)SO2
D)O3
E)Pb

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
53
Which of these processes is represented by the equation?
C6H12O6 + 6O2 → 6CO2 + 6H2O + energy
A)Nitrogen fixation
B)Combustion
C)Photosynthesis
D)Respiration
E)Ozone depletion
C6H12O6 + 6O2 → 6CO2 + 6H2O + energy
A)Nitrogen fixation
B)Combustion
C)Photosynthesis
D)Respiration
E)Ozone depletion

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
54
Which of these pollutants is produced primarily by forest fires, power plants, industries and automobile exhaust?
A)SO2
B)PM-2.5
C)CO
D)O3
E)Pb
A)SO2
B)PM-2.5
C)CO
D)O3
E)Pb

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
55
Which of is not a risk factor of excessive exposure to UV-B rays?
A)Skin cancer
B)Premature aging
C)Cataracts
D)Depressed immune system
E)All of these are risk factors.
A)Skin cancer
B)Premature aging
C)Cataracts
D)Depressed immune system
E)All of these are risk factors.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
56
18 ppm equals
A)0.18%
B)0.018%
C)0.0018%
D)0.00018%
A)0.18%
B)0.018%
C)0.0018%
D)0.00018%

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
57
Which of these processes is represented by the equation?
6H2O + 6CO2 + energy → C6H12O6 + 6O2
A)Nitrogen fixation
B)Combustion
C)Photosynthesis
D)Respiration
E)Ozone depletion
6H2O + 6CO2 + energy → C6H12O6 + 6O2
A)Nitrogen fixation
B)Combustion
C)Photosynthesis
D)Respiration
E)Ozone depletion

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
58
Which of these substances is \are not important in the life-cycle of plants?
I. N2
II. O2
III. H2O
IV. CO2
A)I only
B)II only
C)II and III
D)I and II
E)I, III, and IV
I. N2
II. O2
III. H2O
IV. CO2
A)I only
B)II only
C)II and III
D)I and II
E)I, III, and IV

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
59
The "PM" in the abbreviation PM-2.5 stands for?
A)Primary mist
B)Pollutant material
C)Particulate matter
D)Particles of metal
E)None of the above
A)Primary mist
B)Pollutant material
C)Particulate matter
D)Particles of metal
E)None of the above

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
60
Which of these gases is \are not a part (either as a reactant or as a product)of the biochemical reaction known as photosynthesis?
I. N2
II. O2
III. H2O
IV. CO2
A)I only
B)I and III
C)II and III
D)I and IV
E)I, III, and IV
I. N2
II. O2
III. H2O
IV. CO2
A)I only
B)I and III
C)II and III
D)I and IV
E)I, III, and IV

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
61
Which of these is the correct ordering of the regions of the atmosphere from lowest altitude to highest altitude?
A)ionosphere < mesosphere < troposphere < stratosphere
B)stratosphere < ionosphere < troposphere < mesosphere
C)troposphere < stratosphere < mesosphere < ionosphere
D)troposphere < mesosphere < stratosphere < ionosphere
E)mesosphere < troposphere < stratosphere < ionosphere
A)ionosphere < mesosphere < troposphere < stratosphere
B)stratosphere < ionosphere < troposphere < mesosphere
C)troposphere < stratosphere < mesosphere < ionosphere
D)troposphere < mesosphere < stratosphere < ionosphere
E)mesosphere < troposphere < stratosphere < ionosphere

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
62
Which of these compounds is credited with the accelerated depletion of the ozone layer?
A)CO
B)CFCs
C)O2
D)NO
E)SO2
A)CO
B)CFCs
C)O2
D)NO
E)SO2

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
63
Which of these elements is the reason that chemically decomposed ozone will not reform?
A)Carbon
B)Fluorine
C)Oxygen
D)Chlorine
E)Nitrogen
A)Carbon
B)Fluorine
C)Oxygen
D)Chlorine
E)Nitrogen

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
64
Which of these processes is represented by the equation?
O3 + UV light → O + O2
A)UV absorption
B)Combustion
C)Photosynthesis
D)Respiration
E)Nitrogen fixation
O3 + UV light → O + O2
A)UV absorption
B)Combustion
C)Photosynthesis
D)Respiration
E)Nitrogen fixation

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
65
Which of these regions has the most significant ozone depletion?
A)North America
B)Eastern Europe
C)Antarctica
D)Middle East
E)Southern Africa
A)North America
B)Eastern Europe
C)Antarctica
D)Middle East
E)Southern Africa

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
66
Which of these compounds replaced CFCs used in refrigeration?
A)HCFCs
B)Freon-12
C)CO2
D)N2
E)silicon
A)HCFCs
B)Freon-12
C)CO2
D)N2
E)silicon

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
67
Which of these is not a source of chlorofluorocarbons?
A)Aerosol propellants
B)Refrigeration systems
C)Industrial solvents
D)Fast-food containers
E)Automobile exhaust
A)Aerosol propellants
B)Refrigeration systems
C)Industrial solvents
D)Fast-food containers
E)Automobile exhaust

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck


