Deck 2: The Chemical Context of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/83
Play
Full screen (f)
Deck 2: The Chemical Context of Life
1
Molybdenum has an atomic number of 42. Several common isotopes exist, with mass numbers of 92, 94, 95, 96, 97, 98, and 100. Therefore, which of the following can be true?
A) Molybdenum atoms can have between 50 and 58 neutrons.
B) The isotopes of molybdenum have different electron configurations.
C) The isotopes of molybdenum can have between 50 and 58 protons.
D) The isotopes of molybdenum have between 50 and 58 neutrons and have different electron configurations.
E) The isotopes of molybdenum have between 50 and 58 protons and have different electron configurations.
A) Molybdenum atoms can have between 50 and 58 neutrons.
B) The isotopes of molybdenum have different electron configurations.
C) The isotopes of molybdenum can have between 50 and 58 protons.
D) The isotopes of molybdenum have between 50 and 58 neutrons and have different electron configurations.
E) The isotopes of molybdenum have between 50 and 58 protons and have different electron configurations.
A
2
Why is each element unique and different from other elements in chemical properties?
A) Each element has a unique atomic mass.
B) Each element has a unique atomic weight.
C) Each element has a unique number of protons in its nucleus.
D) Each element has a unique number of neutrons in its nucleus.
E) Each element has different radioactive properties.
A) Each element has a unique atomic mass.
B) Each element has a unique atomic weight.
C) Each element has a unique number of protons in its nucleus.
D) Each element has a unique number of neutrons in its nucleus.
E) Each element has different radioactive properties.
C
3
Which of the following statements is false?
A) Carbon, hydrogen, oxygen, and nitrogen are the most abundant elements of living matter.
B) Some trace elements are very abundant on Earth.
C) Virtually all organisms require the same elements in the same quantities.
D) Iron is an example of an element needed by all organisms.
E) Other than some trace elements, animals are mostly made up of the same elements as plants, in similar proportions.
A) Carbon, hydrogen, oxygen, and nitrogen are the most abundant elements of living matter.
B) Some trace elements are very abundant on Earth.
C) Virtually all organisms require the same elements in the same quantities.
D) Iron is an example of an element needed by all organisms.
E) Other than some trace elements, animals are mostly made up of the same elements as plants, in similar proportions.
C
4
What factors are most important in determining which elements are most common in living matter?
A) the relative abundances of the elements in Earth's crust and atmosphere
B) the emergent properties of the simple compounds made from these elements
C) the reactivity of the elements with water
D) the chemical stability of the elements
E) both the relative abundances of the elements and the emergent properties of the compounds made from these elements
A) the relative abundances of the elements in Earth's crust and atmosphere
B) the emergent properties of the simple compounds made from these elements
C) the reactivity of the elements with water
D) the chemical stability of the elements
E) both the relative abundances of the elements and the emergent properties of the compounds made from these elements

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
5
Atoms whose outer electron shells contain 8 electrons tend to
A) form ions in aqueous solutions.
B) form hydrogen bonds in aqueous solutions.
C) be stable and chemically nonreactive, or inert.
D) be gaseous at room temperature.
E) be both chemically inert and gaseous at room temperature.
A) form ions in aqueous solutions.
B) form hydrogen bonds in aqueous solutions.
C) be stable and chemically nonreactive, or inert.
D) be gaseous at room temperature.
E) be both chemically inert and gaseous at room temperature.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
6
Knowing just the atomic mass of an element allows inferences about which of the following?
A) the chemical properties of the element
B) the number of protons in the element
C) the number of neutrons in the element
D) the number of protons plus neutrons in the element
E) both the number of protons and the chemical properties of the element
A) the chemical properties of the element
B) the number of protons in the element
C) the number of neutrons in the element
D) the number of protons plus neutrons in the element
E) both the number of protons and the chemical properties of the element

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
7
The nucleus of a nitrogen atom contains 7 neutrons and 7 protons. Which of the following is a correct statement concerning nitrogen?
A) The nitrogen atom has a mass number of approximately 7 daltons and an atomic mass of 14.
B) The nitrogen atom has a mass number of approximately 14 daltons and an atomic mass of 7.
C) The nitrogen atom has a mass number of 14 and an atomic mass of 7 grams.
D) The nitrogen atom has a mass number of 7 and an atomic number of 14.
E) The nitrogen atom has a mass number of 14 and an atomic mass of approximately 14 daltons.
A) The nitrogen atom has a mass number of approximately 7 daltons and an atomic mass of 14.
B) The nitrogen atom has a mass number of approximately 14 daltons and an atomic mass of 7.
C) The nitrogen atom has a mass number of 14 and an atomic mass of 7 grams.
D) The nitrogen atom has a mass number of 7 and an atomic number of 14.
E) The nitrogen atom has a mass number of 14 and an atomic mass of approximately 14 daltons.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
8
One difference between carbon-12( 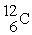 )is that carbon-14(
)is that carbon-14( 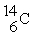 )has
)has
A) two more protons than carbon-12.
B) two more electrons than carbon-12.
C) two more neutrons than carbon-12.
D) two more protons and two more neutrons than carbon-12.
E) two more electrons and two more neutrons than carbon-12.
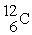 )is that carbon-14(
)is that carbon-14( 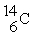 )has
)hasA) two more protons than carbon-12.
B) two more electrons than carbon-12.
C) two more neutrons than carbon-12.
D) two more protons and two more neutrons than carbon-12.
E) two more electrons and two more neutrons than carbon-12.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
9
The precise weight of a mole of some pure elements like silicon (Si)can vary slightly from the standard atomic mass, or even from sample to sample. Why?
A) The element may undergo radioactive decay.
B) The element may react with itself and gain or lose subatomic particles.
C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element.
D) The element may have multiple stable isotopes, and the isotopic composition may vary from sample to sample.
E) The amount of energy absorbed by the element affects the mass of its electrons, and thus the atomic mass can vary slightly.
A) The element may undergo radioactive decay.
B) The element may react with itself and gain or lose subatomic particles.
C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element.
D) The element may have multiple stable isotopes, and the isotopic composition may vary from sample to sample.
E) The amount of energy absorbed by the element affects the mass of its electrons, and thus the atomic mass can vary slightly.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
10
Carbon-12 is the most common isotope of carbon, and has an atomic mass of 12 daltons. A mole of carbon in naturally occurring coal, however, weighs slightly more than 12 grams. Why?
A) The atomic mass does not include the mass of electrons.
B) Some carbon atoms in nature have an extra proton.
C) Some carbon atoms in nature have more neutrons.
D) Some carbon atoms in nature have a different valence electron distribution.
E) Some carbon atoms in nature have undergone radioactive decay.
A) The atomic mass does not include the mass of electrons.
B) Some carbon atoms in nature have an extra proton.
C) Some carbon atoms in nature have more neutrons.
D) Some carbon atoms in nature have a different valence electron distribution.
E) Some carbon atoms in nature have undergone radioactive decay.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
11
Electrons exist only at fixed levels of potential energy. However, if an atom absorbs sufficient energy, a possible result is that
A) an electron may move to an electron shell farther away from the nucleus.
B) an electron may move to an electron shell closer to the nucleus.
C) the atom may become a radioactive isotope.
D) the atom would become a positively charged ion, or cation, and become a radioactive isotope.
E) the atom would become a negatively charged ion, or anion.
A) an electron may move to an electron shell farther away from the nucleus.
B) an electron may move to an electron shell closer to the nucleus.
C) the atom may become a radioactive isotope.
D) the atom would become a positively charged ion, or cation, and become a radioactive isotope.
E) the atom would become a negatively charged ion, or anion.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
12
From its atomic number of 15, it is possible to predict that the phosphorus atom has
A) 15 neutrons.
B) 15 protons.
C) 15 electrons.
D) 8 electrons in its outermost electron shell.
E) 15 protons and 15 electrons.
A) 15 neutrons.
B) 15 protons.
C) 15 electrons.
D) 8 electrons in its outermost electron shell.
E) 15 protons and 15 electrons.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following best describes the relationship between the atoms described below?

A) They are isomers.
B) They are polymers.
C) They are isotopes.
D) They contain 1 and 3 protons, respectively.
E) They each contain 1 neutron.

A) They are isomers.
B) They are polymers.
C) They are isotopes.
D) They contain 1 and 3 protons, respectively.
E) They each contain 1 neutron.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
14
In what way are elements in the same column of the periodic table the same?
A) They have the same number of protons.
B) They have the same number of neutrons.
C) They have the same number of electrons.
D) They have the same number of electrons in their valence shell.
E) They have the same number of electron shells.
A) They have the same number of protons.
B) They have the same number of neutrons.
C) They have the same number of electrons.
D) They have the same number of electrons in their valence shell.
E) They have the same number of electron shells.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
15
Oxygen has an atomic number of 8 and a mass number of 16. Thus, what is the atomic mass of an oxygen atom?
A) exactly 8 grams
B) exactly 8 daltons
C) approximately 16 grams
D) approximately 16 daltons
E) 24 amu (atomic mass units)
A) exactly 8 grams
B) exactly 8 daltons
C) approximately 16 grams
D) approximately 16 daltons
E) 24 amu (atomic mass units)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
16
An atom has 6 electrons in its outer shell. How many unpaired electrons does it have?
A) 0
B) 2
C) 4
D) 6
E) 2 or 4
A) 0
B) 2
C) 4
D) 6
E) 2 or 4

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
17
Trace elements are those required by an organism in only minute quantities. Which of the following is a trace element that is required by humans and other vertebrates, but not by other organisms such as bacteria or plants?
A) nitrogen
B) calcium
C) iodine
D) sodium
E) phosphorus
A) nitrogen
B) calcium
C) iodine
D) sodium
E) phosphorus

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
18
The atomic number of nitrogen is 7. Nitrogen-15 is heavier than nitrogen-14 because the atomic nucleus of nitrogen-15 contains how many neutrons?
A) 6
B) 7
C) 8
D) 12
E) 14
A) 6
B) 7
C) 8
D) 12
E) 14

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
19
The atomic number of neon is 10. Therefore, which of the following is most correct about an atom of neon?
A) It has 8 electrons in its outer electron shell.
B) It is inert.
C) It has an atomic mass of 10 daltons.
D) It has 8 electrons in its outer electron shell and it is inert.
E) It has 8 electrons in its outer electron shell, it is inert, and it has an atomic mass of 10 daltons.
A) It has 8 electrons in its outer electron shell.
B) It is inert.
C) It has an atomic mass of 10 daltons.
D) It has 8 electrons in its outer electron shell and it is inert.
E) It has 8 electrons in its outer electron shell, it is inert, and it has an atomic mass of 10 daltons.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
20
About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living matter?
A) carbon, sodium, hydrogen, nitrogen
B) carbon, oxygen, phosphorus, hydrogen
C) oxygen, hydrogen, calcium, nitrogen
D) carbon, hydrogen, nitrogen, oxygen
E) carbon, oxygen, nitrogen, calcium
A) carbon, sodium, hydrogen, nitrogen
B) carbon, oxygen, phosphorus, hydrogen
C) oxygen, hydrogen, calcium, nitrogen
D) carbon, hydrogen, nitrogen, oxygen
E) carbon, oxygen, nitrogen, calcium

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
21
What is the difference between covalent bonds and ionic bonds?
A) Covalent bonds are formed between atoms to form molecules; ionic bonds are formed between atoms to form compounds.
B) Covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms.
C) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between atoms.
D) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between atoms.
E) Covalent bonds involve the transfer of electrons between atoms; ionic bonds involve the sharing of electrons between atoms.
A) Covalent bonds are formed between atoms to form molecules; ionic bonds are formed between atoms to form compounds.
B) Covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms.
C) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between atoms.
D) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between atoms.
E) Covalent bonds involve the transfer of electrons between atoms; ionic bonds involve the sharing of electrons between atoms.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following molecules contains the most polar covalent bond?
A) H₂
B) O₂
C) CO₂
D) H₂O
E) CH₄
A) H₂
B) O₂
C) CO₂
D) H₂O
E) CH₄

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
23
What is the maximum number of electrons in a single 2 p orbital of an atom?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
24
What is the maximum number of covalent bonds an element with atomic number 8 can make with hydrogen?
A) 1
B) 2
C) 3
D) 4
E) 6
A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
25
Two atoms appear to have the same mass number. These atoms
A) must have the same atomic number.
B) must have the same number of electrons.
C) must have the same chemical properties.
D) must have the same number of protons + neutrons.
E) must have the same atomic number, the same number of protons + neutrons, the same number of electrons, and the same chemical properties.
A) must have the same atomic number.
B) must have the same number of electrons.
C) must have the same chemical properties.
D) must have the same number of protons + neutrons.
E) must have the same atomic number, the same number of protons + neutrons, the same number of electrons, and the same chemical properties.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
26
The atomic number of chlorine is 17. The atomic number of magnesium is 12. What is the formula for magnesium chloride?
A) MgCl
B) MgCl₂
C) Mg₂Cl
D) Mg₂Cl₂
E) MgCl₃
A) MgCl
B) MgCl₂
C) Mg₂Cl
D) Mg₂Cl₂
E) MgCl₃

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
27
The organic molecules in living organisms have a measurably lower ratio of carbon-13/carbon-12, two stable isotopes of carbon that comprise approximately 1.1% and 98.9% of atmospheric carbon, respectively. What is a reasonable explanation for this phenomenon?
A) Photosynthesis preferentially uses carbon dioxide molecules with carbon-12, and the lower carbon-13/carbon-12 ratio propagates through the food chain.
B) Carbon dioxide molecules with carbon-13 stay in the upper atmosphere and are less available to terrestrial plants and algae.
C) Carbon-13 has a different valence electron configuration and is therefore less chemically reactive than carbon-12.
D) Oxygen atoms preferentially react with carbon-13, thereby enriching the atmosphere with carbon dioxide molecules containing carbon-13 atoms.
E) Carbon dioxide molecules containing carbon-13 are heavier and sink into the ocean depths, making them less available to living organisms.
A) Photosynthesis preferentially uses carbon dioxide molecules with carbon-12, and the lower carbon-13/carbon-12 ratio propagates through the food chain.
B) Carbon dioxide molecules with carbon-13 stay in the upper atmosphere and are less available to terrestrial plants and algae.
C) Carbon-13 has a different valence electron configuration and is therefore less chemically reactive than carbon-12.
D) Oxygen atoms preferentially react with carbon-13, thereby enriching the atmosphere with carbon dioxide molecules containing carbon-13 atoms.
E) Carbon dioxide molecules containing carbon-13 are heavier and sink into the ocean depths, making them less available to living organisms.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
28
A covalent bond is likely to be polar when
A) one of the atoms sharing electrons is much more electronegative than the other atom.
B) the two atoms sharing electrons are equally electronegative.
C) oxygen is one of the two atoms sharing electrons.
D) one of the atoms has absorbed more energy than the other atom.
E) the two atoms sharing electrons are different elements.
A) one of the atoms sharing electrons is much more electronegative than the other atom.
B) the two atoms sharing electrons are equally electronegative.
C) oxygen is one of the two atoms sharing electrons.
D) one of the atoms has absorbed more energy than the other atom.
E) the two atoms sharing electrons are different elements.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
29
Phosphorus-32, a radioactive isotope of phosphorus-31 (atomic number 15), undergoes a form of radioactive decay whereby a neutron turns into a proton and emits radiation in the form of an electron. What is the product of such radioactive decay of phosphorus-32?
A) phosphorus-31
B) a positively charged phosphorus-31 ion
C) a negatively charged phosphorus-32 ion
D) sulfur-32 (atomic number 16)
E) the conversion of the phosphorus-32 atom into pure energy
A) phosphorus-31
B) a positively charged phosphorus-31 ion
C) a negatively charged phosphorus-32 ion
D) sulfur-32 (atomic number 16)
E) the conversion of the phosphorus-32 atom into pure energy

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
30
When two atoms are equally electronegative, they will interact to form
A) hydrogen bonds.
B) van der Waals interactions.
C) polar covalent bonds.
D) nonpolar covalent bonds.
E) ionic bonds.
A) hydrogen bonds.
B) van der Waals interactions.
C) polar covalent bonds.
D) nonpolar covalent bonds.
E) ionic bonds.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
31
In ammonium chloride salt (NH₄Cl)the anion is a single chloride ion, Cl. What is the cation of NH₄Cl?
A) N, with a charge of +1
B) NH, with a charge of +1
C) H₃, with a charge of +1
D) NH₄, with a charge of +1
E) NH₄, with a charge of +4
A) N, with a charge of +1
B) NH, with a charge of +1
C) H₃, with a charge of +1
D) NH₄, with a charge of +1
E) NH₄, with a charge of +4

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
32
Nitrogen (N)is much more electronegative than hydrogen (H). Which of the following statements is correct about the atoms in ammonia (NH₃)?
A) Each hydrogen atom has a partial positive charge; the nitrogen atom has a partial negative charge.
B) The nitrogen atom has a strong positive charge; each hydrogen atom has a strong positive charge.
C) Each hydrogen atom has a slight negative charge; the nitrogen atom has a strong positive charge.
D) The nitrogen atom has a slight positive charge; each hydrogen atom has a slight negative charge.
E) There are covalent bonds between the hydrogen atoms and polar bonds between each hydrogen atom and the nitrogen atom.
A) Each hydrogen atom has a partial positive charge; the nitrogen atom has a partial negative charge.
B) The nitrogen atom has a strong positive charge; each hydrogen atom has a strong positive charge.
C) Each hydrogen atom has a slight negative charge; the nitrogen atom has a strong positive charge.
D) The nitrogen atom has a slight positive charge; each hydrogen atom has a slight negative charge.
E) There are covalent bonds between the hydrogen atoms and polar bonds between each hydrogen atom and the nitrogen atom.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
33
In comparing covalent bonds and ionic bonds, which of the following would you expect?
A) An atom can form covalent bonds with multiple partner atoms, but only a single ionic bond with a single partner atom.
B) Covalent bonds and ionic bonds occupy opposite ends of a continuous spectrum, from nearly equal to completely unequal sharing of electrons.
C) Both involve electrical attraction between the electrons of one atom and the nucleus of the other atom.
D) Ionic interactions remain when covalent bonds are broken in water. Ionic bonds are much stronger than covalent bonds.
A) An atom can form covalent bonds with multiple partner atoms, but only a single ionic bond with a single partner atom.
B) Covalent bonds and ionic bonds occupy opposite ends of a continuous spectrum, from nearly equal to completely unequal sharing of electrons.
C) Both involve electrical attraction between the electrons of one atom and the nucleus of the other atom.
D) Ionic interactions remain when covalent bonds are broken in water. Ionic bonds are much stronger than covalent bonds.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
34
An atom with atomic number 12 would have what type of chemical behavior in bonding with other elements?
A) It would form ions with a +1 charge.
B) It would form ions with a +2 charge.
C) It would form ions with a -1 charge.
D) It would form ions with a -2 charge.
E) It would form two covalent bonds with other atoms.
A) It would form ions with a +1 charge.
B) It would form ions with a +2 charge.
C) It would form ions with a -1 charge.
D) It would form ions with a -2 charge.
E) It would form two covalent bonds with other atoms.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
35
Fluorine has an atomic number of 9 and a mass number of 19. How many electrons are needed to complete the valence shell of a fluorine atom?
A) 1
B) 3
C) 0
D) 7
E) 9
A) 1
B) 3
C) 0
D) 7
E) 9

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
36
The atomic number of each atom is given to the left of each of the elements below. Which of the atoms has the same valence as carbon ( 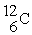 )?
)?
A) ₇N nitrogen
B) ₉F flourine
C) ₁₀Ne neon
D) ₁₂Mg magnesium
E) ₁₄Si silicon
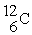 )?
)?A) ₇N nitrogen
B) ₉F flourine
C) ₁₀Ne neon
D) ₁₂Mg magnesium
E) ₁₄Si silicon

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
37
What results from an unequal sharing of electrons between atoms?
A) a nonpolar covalent bond
B) a polar covalent bond
C) an ionic bond
D) a hydrogen bond
E) a hydrophobic interaction
A) a nonpolar covalent bond
B) a polar covalent bond
C) an ionic bond
D) a hydrogen bond
E) a hydrophobic interaction

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
38
If an atom of sulfur (atomic number 16)were allowed to react with atoms of hydrogen (atomic number 1), which of the molecules below would be formed?
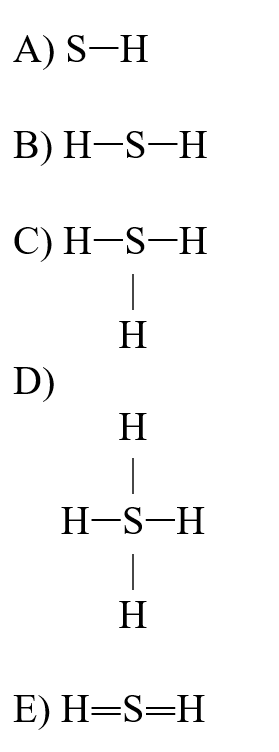


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
39
If a salamander relied on hydrogen bonds to cling to surfaces, what type of surface would cause the most problems for this animal?
A) a surface coated with a thin film of water
B) a surface made with carbon and hydrogen atoms covalently bonded together
C) a surface made with carbon, hydrogen, and oxygen atoms covalently bonded together
D) a surface made with carbon, hydrogen, nitrogen, and oxygen atoms covalently bonded together
E) a surface made with silicon and oxygen atoms covalently bonded together
A) a surface coated with a thin film of water
B) a surface made with carbon and hydrogen atoms covalently bonded together
C) a surface made with carbon, hydrogen, and oxygen atoms covalently bonded together
D) a surface made with carbon, hydrogen, nitrogen, and oxygen atoms covalently bonded together
E) a surface made with silicon and oxygen atoms covalently bonded together

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
40
A covalent chemical bond is one in which
A) electrons are removed from one atom and transferred to another atom so that the two atoms become oppositely charged.
B) protons and neutrons are shared by two atoms so as to satisfy the requirements of both atoms.
C) outer-shell electrons of two atoms are shared so as to satisfactorily fill the outer electron shells of both atoms.
D) outer-shell electrons of one atom are transferred to fill the inner electron shell of another atom.
E) an electron occupies a hybrid orbital located between the nuclei of two atoms.
A) electrons are removed from one atom and transferred to another atom so that the two atoms become oppositely charged.
B) protons and neutrons are shared by two atoms so as to satisfy the requirements of both atoms.
C) outer-shell electrons of two atoms are shared so as to satisfactorily fill the outer electron shells of both atoms.
D) outer-shell electrons of one atom are transferred to fill the inner electron shell of another atom.
E) an electron occupies a hybrid orbital located between the nuclei of two atoms.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
41
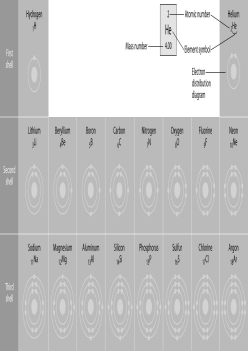
Refer to the figure above (first three rows of the periodic table). If life arose on a planet where carbon is absent, which element might fill the role of carbon?
A) boron
B) silicon
C) nitrogen
D) aluminum
E) phosphorus

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
42
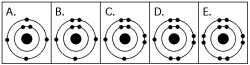
Which drawing in the figure above is of the electron configuration of a sodium ₁₁Na⁺ ion?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following correctly describes chemical equilibrium?
A) Forward and reverse reactions continue with no effect on the concentrations of the reactants and products.
B) Concentrations of products are higher than the concentrations of the reactants.
C) Forward and reverse reactions have stopped so that the concentration of the reactants equals the concentration of the products.
D) Reactions stop only when all reactants have been converted to products.
E) There are equal concentrations of reactants and products, and the reactions have stopped.
A) Forward and reverse reactions continue with no effect on the concentrations of the reactants and products.
B) Concentrations of products are higher than the concentrations of the reactants.
C) Forward and reverse reactions have stopped so that the concentration of the reactants equals the concentration of the products.
D) Reactions stop only when all reactants have been converted to products.
E) There are equal concentrations of reactants and products, and the reactions have stopped.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
44

Which drawing in the figure above depicts the electron configuration of an atom capable of forming three covalent bonds with other atoms?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is true for this reaction?
3 H₂ + N₂ ↔ 2 NH₃
A) The reaction is nonreversible.
B) Hydrogen and nitrogen are the reactants of the reverse reaction.
C) Hydrogen and nitrogen are the products of the forward reaction.
D) Ammonia is being formed and decomposed.
E) Hydrogen and nitrogen are being decomposed.
3 H₂ + N₂ ↔ 2 NH₃
A) The reaction is nonreversible.
B) Hydrogen and nitrogen are the reactants of the reverse reaction.
C) Hydrogen and nitrogen are the products of the forward reaction.
D) Ammonia is being formed and decomposed.
E) Hydrogen and nitrogen are being decomposed.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
46
How many electron pairs are shared between carbon atoms in a molecule that has the formula C₂H₄?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following would be regarded as compounds?
A) H₂O, O₂, and CH₄
B) H₂O and O₂
C) O₂ and CH₄
D) CH₄ and O₂, but not H₂O
E) H₂O and CH₄, but not O₂
A) H₂O, O₂, and CH₄
B) H₂O and O₂
C) O₂ and CH₄
D) CH₄ and O₂, but not H₂O
E) H₂O and CH₄, but not O₂

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
48
What is the maximum number of hydrogen atoms that can be covalently bonded in a molecule containing two carbon atoms?
A) 2
B) 3
C) 4
D) 6
E) 8
A) 2
B) 3
C) 4
D) 6
E) 8

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
49
What bonding or interaction is most likely to occur among a broad array of molecules of various types (polar, nonpolar, hydrophilic, hydrophobic)?
A) covalent bonding
B) polar covalent bonding
C) ionic bonding
D) hydrogen bonding
E) van der Waals interactions
A) covalent bonding
B) polar covalent bonding
C) ionic bonding
D) hydrogen bonding
E) van der Waals interactions

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
50

Which drawing in the figure above depicts the electron configuration of an atom that can form covalent bonds with two hydrogen atoms?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
51
Which bond or interaction would be difficult to disrupt when compounds are put into water?
A) covalent bond
B) hydrogen bond
C) van der Waals interaction
D) ionic bond
E) either covalent bonds or ionic bonds
A) covalent bond
B) hydrogen bond
C) van der Waals interaction
D) ionic bond
E) either covalent bonds or ionic bonds

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
52

Which drawing in the figure above depicts the electron configuration of an element with chemical properties most similar to Helium (₂He)?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
53

Which drawing in the figure above depicts an atom with a valence of 2?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is not considered to be a weak molecular interaction?
A) a covalent bond
B) a van der Waals interaction
C) an ionic bond in the presence of water
D) a hydrogen bond
E) both a hydrogen bond and a covalent bond
A) a covalent bond
B) a van der Waals interaction
C) an ionic bond in the presence of water
D) a hydrogen bond
E) both a hydrogen bond and a covalent bond

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following correctly describes any reaction that has reached chemical equilibrium?
A) The concentration of the reactants equals the concentration of the products.
B) The rate of the forward reaction is equal to the rate of the reverse reaction.
C) All of the reactants have been converted to the products of the reaction.
D) All of the products have been converted to the reactants of the reaction.
E) Both the forward and the reverse reactions have stopped with no net effect on the concentration of the reactants and the products.
A) The concentration of the reactants equals the concentration of the products.
B) The rate of the forward reaction is equal to the rate of the reverse reaction.
C) All of the reactants have been converted to the products of the reaction.
D) All of the products have been converted to the reactants of the reaction.
E) Both the forward and the reverse reactions have stopped with no net effect on the concentration of the reactants and the products.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
56

Which drawing in the figure above depicts the most electronegative atom?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
57
Van der Waals interactions result when
A) hybrid orbitals overlap.
B) electrons are not symmetrically distributed in a molecule.
C) molecules held by ionic bonds react with water.
D) two polar covalent bonds react.
E) a hydrogen atom loses an electron.
A) hybrid orbitals overlap.
B) electrons are not symmetrically distributed in a molecule.
C) molecules held by ionic bonds react with water.
D) two polar covalent bonds react.
E) a hydrogen atom loses an electron.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
58
Which of these systems is least likely to be at chemical equilibrium?
A) a test tube of living cells
B) a test tube of organic molecules, kept in the freezer
C) a test tube of dry organic molecules, kept at room temperature
D) a test tube of organic molecules dissolved in water, kept at room temperature
E) a test tube of dead cells in water, kept at room temperature
A) a test tube of living cells
B) a test tube of organic molecules, kept in the freezer
C) a test tube of dry organic molecules, kept at room temperature
D) a test tube of organic molecules dissolved in water, kept at room temperature
E) a test tube of dead cells in water, kept at room temperature

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following explains most specifically the attraction of water molecules to one another?
A) nonpolar covalent bond
B) polar covalent bond
C) ionic bond
D) hydrogen bond
E) hydrophobic interaction
A) nonpolar covalent bond
B) polar covalent bond
C) ionic bond
D) hydrogen bond
E) hydrophobic interaction

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
60

Which drawing in the figure above depicts an atom with a valence of 3?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
61
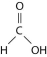
The illustration above shows a representation of formic acid. A formic acid molecule
A) will form hydrogen bonds with water molecules.
B) has a tetrahedral configuration of hybrid electron orbitals for the carbon atom.
C) consists of largely nonpolar covalent bonds.
D) is held together by hydrogen bonds.
E) has a tetrahedral shape and will form hydrogen bonds with water molecules.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
62
How many electrons does an atom of sulfur have in its valence shell (see the figure above)?
A) 4
B) 6
C) 8
D) 16
E) 32

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
63
Which one of the atoms shown would be most likely to form an anion with a charge of -1?
A)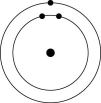
B)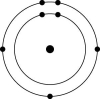
C)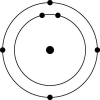
D)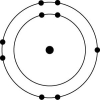
E)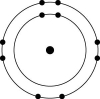
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
64
Which one of the atoms shown would be most likely to form a cation with a charge of +1?
A)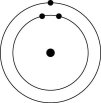
B)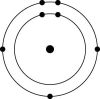
C)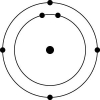
D)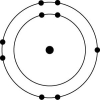
E)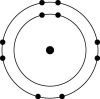
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
65
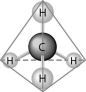
What causes the shape of the molecule shown above?
A) the configuration of the 2 p orbitals in the carbon atom
B) the configuration of the 1 s orbital in the carbon atom
C) the configuration of the sp hybrid orbitals of the electrons shared between the carbon and hydrogen atoms
D) the packing of the carbon and hydrogen atoms in a crystal lattice
E) hydrogen bonding configurations between the carbon and hydrogen atoms

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
66

How many neutrons are present in the nucleus of a phosphorus-32 (³²P)atom (see the figure above)?
A) 5
B) 15
C) 16
D) 17
E) 32

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
67

In the figure above, how many unpaired electrons does phosphorus have in its valence shell?
A) 15
B) 2
C) 3
D) 7
E) 5

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
68
Which statement is true of all atoms that are anions?
A) The atom has more electrons than protons.
B) The atom has more protons than electrons.
C) The atom has fewer protons than does a neutral atom of the same element.
D) The atom has more neutrons than protons.
E) The net charge is 1-.
A) The atom has more electrons than protons.
B) The atom has more protons than electrons.
C) The atom has fewer protons than does a neutral atom of the same element.
D) The atom has more neutrons than protons.
E) The net charge is 1-.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
69
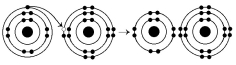
What results from the chemical reaction illustrated above?
A) a cation with a net charge of +1
B) a cation with a net charge of -1
C) an anion with a net charge of +1
D) an anion with a net charge of -1
E) a cation with a net charge of +1 and an anion with a net charge of -1

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
70
The reactivity of an atom arises from
A) the average distance of the outermost electron shell from the nucleus.
B) the existence of unpaired electrons in the valence shell.
C) the sum of the potential energies of all the electron shells.
D) the potential energy of the valence shell.
E) the energy difference between the s and p orbitals.
A) the average distance of the outermost electron shell from the nucleus.
B) the existence of unpaired electrons in the valence shell.
C) the sum of the potential energies of all the electron shells.
D) the potential energy of the valence shell.
E) the energy difference between the s and p orbitals.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
71

In the methane molecule shown in the figure above, bonds have formed that include both the s orbital valence electrons of the hydrogen atoms and the p orbital valence electrons of the carbon. The electron orbitals in these bonds are said to be
A) double orbitals.
B) tetrahedral orbitals.
C) complex orbitals.
D) hybrid orbitals.
E) polar orbitals.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following pairs of atoms would be most likely to form a polar covalent bond?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
73
In the term trace element, the modifier trace means that
A) the element is required in very small amounts.
B) the element can be used as a label to trace atoms through an organism's metabolism.
C) the element is very rare on Earth.
D) the element enhances health but is not essential for the organism's long-term survival.
E) the element passes rapidly through the organism.
A) the element is required in very small amounts.
B) the element can be used as a label to trace atoms through an organism's metabolism.
C) the element is very rare on Earth.
D) the element enhances health but is not essential for the organism's long-term survival.
E) the element passes rapidly through the organism.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
74
What is the atomic number of the cation formed in the reaction illustrated above?
A) 1
B) 8
C) 10
D) 11
E) 16
A) 1
B) 8
C) 10
D) 11
E) 16

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
75
A group of molecular biologists is trying to synthesize a new artificial compound to mimic the effects of a known hormone that influences sexual behavior. They have turned to you for advice. Which of the following compounds is most likely to mimic the effects of the hormone?
A) a compound with the same number of carbon atoms as the hormone
B) a compound with the same molecular mass (measured in daltons) as the hormone
C) a compound with the same three-dimensional shape as part of the hormone
D) a compound with the same number of orbital electrons as the hormone
E) a compound with the same number of hydrogen and nitrogen atoms as the hormone
A) a compound with the same number of carbon atoms as the hormone
B) a compound with the same molecular mass (measured in daltons) as the hormone
C) a compound with the same three-dimensional shape as part of the hormone
D) a compound with the same number of orbital electrons as the hormone
E) a compound with the same number of hydrogen and nitrogen atoms as the hormone

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
76

In the figure above, how many electrons does nitrogen have in its valence shell?
A) 2
B) 5
C) 7
D) 8
E) 14

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
77
Compared with ³¹P, the radioactive isotope ³²P has
A) a different atomic number.
B) a different charge.
C) one more proton.
D) one more electron.
E) one more neutron.
A) a different atomic number.
B) a different charge.
C) one more proton.
D) one more electron.
E) one more neutron.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
78

Based on electron configuration, which of these elements in the figure above would exhibit a chemical behavior most like that of oxygen?
A) carbon
B) hydrogen
C) nitrogen
D) sulfur
E) phosphorus

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following pairs of atoms would be most likely to form an ionic bond?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following statements correctly describes any chemical reaction that has reached equilibrium?
A) The concentrations of products and reactants are equal.
B) The reaction is now irreversible.
C) Both forward and reverse reactions have halted.
D) The rates of the forward and reverse reactions are equal.
E) No reactants remain.
A) The concentrations of products and reactants are equal.
B) The reaction is now irreversible.
C) Both forward and reverse reactions have halted.
D) The rates of the forward and reverse reactions are equal.
E) No reactants remain.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck


