Deck 7: Atoms and Spectra
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/73
Play
Full screen (f)
Deck 7: Atoms and Spectra
1
An atom that has gained one or more electrons is called an isotope.
False
2
In blackbody radiation, short-wavelength and long-wavelength photons are rare.
True
3
The binding energy is the amount of energy ____.
A) needed to pull a proton completely away from the nucleus
B) needed to pull an electron completely away from the nucleus
C) needed to push a neutron into the nucleus
D) needed to completely separate all particles in the atom
E) necessary to move a proton to a permitted orbit
A) needed to pull a proton completely away from the nucleus
B) needed to pull an electron completely away from the nucleus
C) needed to push a neutron into the nucleus
D) needed to completely separate all particles in the atom
E) necessary to move a proton to a permitted orbit
B
4
An atom that has lost or gained one or more electrons is called a(n) ____.
A) ion
B) isotope
C) nucleus
D) molecule
E) electron
A) ion
B) isotope
C) nucleus
D) molecule
E) electron

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
5
Atoms are mostly empty space.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
6
The amount of energy needed to pull an electron completely away from the nucleus is called the Coulomb energy.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
7
The lowest permitted energy level of an atom is called the neutral state.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
8
Most of the mass of an atom is ____.
A) in the electron cloud
B) spread evenly through the atom
C) made up of electrons
D) concentrated in the nucleus
E) concentrated in a disk around the nucleus
A) in the electron cloud
B) spread evenly through the atom
C) made up of electrons
D) concentrated in the nucleus
E) concentrated in a disk around the nucleus

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
9
An absorption spectrum is created when blackbody radiation passes through a cool gas.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
10
The Kelvin temperature scale is based on the freezing point of water.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
11
A neutral atom must have ____.
A) an equal number of protons and neutrons
B) an equal number of protons and electrons
C) an equal number of neutrons and electrons
D) less electrons than protons
E) more electrons than neutrons
A) an equal number of protons and neutrons
B) an equal number of protons and electrons
C) an equal number of neutrons and electrons
D) less electrons than protons
E) more electrons than neutrons

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
12
If absorption lines of sodium are not present in a star's spectrum, the star must not contain any sodium.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
13
If you move an electron from the ground state to a higher energy level, the atom becomes an excited atom.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
14
A continuous spectrum is created by a hot ionized gas.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
15
A hot object that is glowing orange will become redder as it cools.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
16
The particle in an atom that carries a negative charge is the ____.
A) nucleon
B) electron
C) negatron
D) proton
E) neutron
A) nucleon
B) electron
C) negatron
D) proton
E) neutron

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
17
A certain type of atom can only absorb certain wavelengths.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
18
The number of protons in the nucleus determines which element it is.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
19
Small differences in temperature between two stars produce small differences in the amount of energy they radiate.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
20
The agitation of atoms in a hot body creates a continuous spectrum of radiation.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
21
The type of elements present in a gas can be determined by studying the ____.
A) number of ionized atoms in the gas
B) energy of the electrons being emitted by the gas
C) number of photons being absorbed or emitted by the gas
D) wavelengths of photons absorbed or emitted from the gas
E) number of excited atoms in the gas
A) number of ionized atoms in the gas
B) energy of the electrons being emitted by the gas
C) number of photons being absorbed or emitted by the gas
D) wavelengths of photons absorbed or emitted from the gas
E) number of excited atoms in the gas

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
22
A spectrum that displays a smooth variation in intensity of all wavelengths without any breaks is a(n) ____ spectrum.
A) absorption
B) emission
C) continuous
D) Doppler
E) ionization
A) absorption
B) emission
C) continuous
D) Doppler
E) ionization

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
23
The star Betelgeuse appears red; the star Rigel appears blue. What accounts for this difference?
A) Betelgeuse and Rigel have different chemical compositions.
B) Betelgeuse is moving away from Earth, Rigel is moving toward Earth.
C) Betelgeuse is moving toward Earth, Rigel is moving away from Earth.
D) The surface of Betelgeuse is hotter than the surface of Rigel.
E) The surface of Betelgeuse is cooler than the surface of Rigel.
A) Betelgeuse and Rigel have different chemical compositions.
B) Betelgeuse is moving away from Earth, Rigel is moving toward Earth.
C) Betelgeuse is moving toward Earth, Rigel is moving away from Earth.
D) The surface of Betelgeuse is hotter than the surface of Rigel.
E) The surface of Betelgeuse is cooler than the surface of Rigel.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
24
Atoms that have the same number of protons but a different number of neutrons are called ____.
A) ions
B) elements
C) isotopes
D) molecules
E) nucleons
A) ions
B) elements
C) isotopes
D) molecules
E) nucleons

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
25
The type of element is determined by the number of ____.
A) protons in the atom
B) neutrons in the atom
C) electrons in the atom
D) electrons that have been removed from the atom
E) protons that have been removed from the atom
A) protons in the atom
B) neutrons in the atom
C) electrons in the atom
D) electrons that have been removed from the atom
E) protons that have been removed from the atom

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
26
Photons of light can be absorbed by an atom of an element ____.
A) if they match one of several possible wavelengths that are absorbed by that element
B) if they match one of several possible wavelengths that are absorbed by all elements
C) if they match the only particular wavelength that can be absorbed by that element
D) if they match the only particular wavelength that can be absorbed by all elements
E) no matter what wavelength they have
A) if they match one of several possible wavelengths that are absorbed by that element
B) if they match one of several possible wavelengths that are absorbed by all elements
C) if they match the only particular wavelength that can be absorbed by that element
D) if they match the only particular wavelength that can be absorbed by all elements
E) no matter what wavelength they have

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
27
The Stefan-Boltzmann law relates the ____.
A) energy emitted from a surface every second to the temperature of the surface
B) wavelength of peak intensity to the Kelvin temperature
C) binding energy to the Coulomb force
D) difference in energy level to the wavelength of the emitted photon
E) observed wavelength to the velocity of the object
A) energy emitted from a surface every second to the temperature of the surface
B) wavelength of peak intensity to the Kelvin temperature
C) binding energy to the Coulomb force
D) difference in energy level to the wavelength of the emitted photon
E) observed wavelength to the velocity of the object

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
28
An object that acts as a blackbody emits photons because ____.
A) thermal energy excites electrons to the ground state
B) electrons in the atoms of the object jump to lower energy levels
C) electrons in the atoms of the object jump to higher energy levels
D) atoms in the object collide, changing the motion of charged particles
E) atoms in the object have absorbed photons from an external source
A) thermal energy excites electrons to the ground state
B) electrons in the atoms of the object jump to lower energy levels
C) electrons in the atoms of the object jump to higher energy levels
D) atoms in the object collide, changing the motion of charged particles
E) atoms in the object have absorbed photons from an external source

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
29
The amount of energy needed to move an electron from a lower energy level to a higher energy level is the ____.
A) energy of the higher level
B) energy of the lower level
C) difference in energy between the two levels
D) product of the energies of the two levels
E) binding energy
A) energy of the higher level
B) energy of the lower level
C) difference in energy between the two levels
D) product of the energies of the two levels
E) binding energy

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
30
Using Wien's law, you can measure the ____ of a distant object without having to travel to it.
A) binding energy
B) temperature
C) radial motion
D) energy output
E) atomic composition
A) binding energy
B) temperature
C) radial motion
D) energy output
E) atomic composition

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
31
The photons coming from an excited gas create a(n) ____ spectrum.
A) absorption
B) emission
C) continuous
D) Doppler
E) ionization
A) absorption
B) emission
C) continuous
D) Doppler
E) ionization

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
32
If the photons from blackbody radiator radiation pass through a cool gas, a(n) ____ spectrum is produced.
A) absorption
B) emission
C) continuous
D) Doppler
E) ionization
A) absorption
B) emission
C) continuous
D) Doppler
E) ionization

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
33
According to Wien's Law, a(n) ____.
A) hotter object will emit more short wavelength (bluer) radiation
B) hotter object will emit more long wavelength (redder) radiation
C) cooler object will produce less photons
D) cooler object will produce more photons
E) object will produce the same number of photons at different temperatures
A) hotter object will emit more short wavelength (bluer) radiation
B) hotter object will emit more long wavelength (redder) radiation
C) cooler object will produce less photons
D) cooler object will produce more photons
E) object will produce the same number of photons at different temperatures

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
34
The photons coming from blackbody radiation create a(n) ____ spectrum.
A) absorption
B) emission
C) Doppler
D) continuous
E) ionization
A) absorption
B) emission
C) Doppler
D) continuous
E) ionization

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
35
Electromagnetic waves are generated whenever the ____.
A) motion of any particle is changed
B) motion of any charged particle changes
C) motion of a positively charged particle is changed
D) motion of a negatively charged particle is changed
E) acceleration of any charged particle is changed
A) motion of any particle is changed
B) motion of any charged particle changes
C) motion of a positively charged particle is changed
D) motion of a negatively charged particle is changed
E) acceleration of any charged particle is changed

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
36
The arrangement of permitted orbits is ____.
A) the same for each neutron
B) unique for each electron
C) the same for all elements
D) the same for all molecules
E) unique for each element
A) the same for each neutron
B) unique for each electron
C) the same for all elements
D) the same for all molecules
E) unique for each element

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
37
An atom must emit a photon when a(n) ____.
A) electron moves from a lower to a higher energy level
B) proton moves from a lower to a higher energy level
C) proton moves from a higher to a lower energy level
D) electron moves from a higher to a lower energy level
E) atom becomes ionized
A) electron moves from a lower to a higher energy level
B) proton moves from a lower to a higher energy level
C) proton moves from a higher to a lower energy level
D) electron moves from a higher to a lower energy level
E) atom becomes ionized

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
38
As a blackbody becomes hotter it will radiate ____.
A) less energy, at a longer wavelength of maximum intensity
B) less energy, at a shorter wavelength of maximum intensity
C) more energy, at a longer wavelength of maximum intensity
D) more energy, at a shorter wavelength of maximum intensity
E) more energy, at a constant wavelength of maximum intensity
A) less energy, at a longer wavelength of maximum intensity
B) less energy, at a shorter wavelength of maximum intensity
C) more energy, at a longer wavelength of maximum intensity
D) more energy, at a shorter wavelength of maximum intensity
E) more energy, at a constant wavelength of maximum intensity

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
39
Blackbody radiation is caused by an object's ____.
A) chemical structure
B) binding energy
C) excitation level
D) absolute zero
E) thermal agitation
A) chemical structure
B) binding energy
C) excitation level
D) absolute zero
E) thermal agitation

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
40
A spectrum that displays a smooth variation in intensity over a range of wavelengths with breaks where no energy is observed at specific wavelengths is a(n) ____ spectrum.
A) absorption
B) emission
C) continuous
D) Doppler
E) ionization
A) absorption
B) emission
C) continuous
D) Doppler
E) ionization

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
41
An object that is a perfect absorber and emitter of radiation is called a(n) _______________.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
42
The Doppler effect is useful in measuring the ____.
A) distance to an object
B) chemical composition of an object
C) temperature of an object
D) motion of an object across the line of sight of an observer
E) motion of an object toward or away from the observer
A) distance to an object
B) chemical composition of an object
C) temperature of an object
D) motion of an object across the line of sight of an observer
E) motion of an object toward or away from the observer

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
43
Match between columns

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
44
A star like the Sun emits a(n) ____ spectrum.
A) absorption
B) emission
C) continuous
D) Doppler
E) ionization
A) absorption
B) emission
C) continuous
D) Doppler
E) ionization

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
45
The _______________ temperature scale is used in astronomy because it is based on absolute zero.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
46
A shift in the position of an emission line of an element toward the red or blue is produced by ____.
A) atoms that become ionized
B) electrons that transfer from a high energy level to a low energy level
C) electrons that transfer from a low energy level to a high energy level
D) the intensity of agitation of atoms in a hot gas
E) the motion of an object toward or away from the observer
A) atoms that become ionized
B) electrons that transfer from a high energy level to a low energy level
C) electrons that transfer from a low energy level to a high energy level
D) the intensity of agitation of atoms in a hot gas
E) the motion of an object toward or away from the observer

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
47
What is binding energy, and what physical effect causes it?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
48
A spectrum that displays no energy except at very specific wavelengths where intense radiation is observed is a(n) ____ spectrum.
A) absorption
B) emission
C) continuous
D) Doppler
E) ionization
A) absorption
B) emission
C) continuous
D) Doppler
E) ionization

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
49
The wavelength of maximum intensity is useful in measuring the ____.
A) surface temperatures of an object from their colors
B) distance to an object from their colors
C) chemical composition of an object
D) motion of an object across the observer's line of sight
E) motion of an object toward or away from the observer
A) surface temperatures of an object from their colors
B) distance to an object from their colors
C) chemical composition of an object
D) motion of an object across the observer's line of sight
E) motion of an object toward or away from the observer

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
50
Absorption lines are produced by ____.
A) atoms that become ionized
B) electrons that transfer from a high energy level to a low energy level
C) electrons that transfer from a low energy level to a high energy level
D) the intensity of agitation of atoms in a hot gas
E) the motion of an object toward or away from the observer
A) atoms that become ionized
B) electrons that transfer from a high energy level to a low energy level
C) electrons that transfer from a low energy level to a high energy level
D) the intensity of agitation of atoms in a hot gas
E) the motion of an object toward or away from the observer

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
51
An atom can only have orbits of certain sizes, called _______________ orbits.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
52
A continuous spectrum is produced by ____.
A) atoms that become ionized
B) electrons that transfer from a high energy level to a low energy level
C) electrons that transfer from a low energy level to a high energy level
D) the intensity of agitation of atoms in a hot gas
E) the motion of an object toward or away from the observer
A) atoms that become ionized
B) electrons that transfer from a high energy level to a low energy level
C) electrons that transfer from a low energy level to a high energy level
D) the intensity of agitation of atoms in a hot gas
E) the motion of an object toward or away from the observer

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
53
The _______________ is the amount of energy needed to pull an electron completely away from the nucleus.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
54
A(n) _______________ is an atom that has gained or lost one or more electrons.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
55
An astronomer records the spectrum of light coming from a distant star. Describe what information the astronomer can deduce from this stellar spectrum, and what physical principles she would apply.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
56
Physicists and astronomers usually refer to the permitted orbits of an atom as the atom's _______________ levels.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
57
Emission lines are produced by ____.
A) atoms that become ionized
B) electrons that transfer from a high energy level to a low energy level
C) electrons that transfer from a low energy level to a high energy level
D) the intensity of agitation of atoms in a hot gas
E) the motion of an object toward or away from the observer
A) atoms that become ionized
B) electrons that transfer from a high energy level to a low energy level
C) electrons that transfer from a low energy level to a high energy level
D) the intensity of agitation of atoms in a hot gas
E) the motion of an object toward or away from the observer

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
58
What process in an isolated atom will make it absorb a photon?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
59
The position of an absorption or emission line is useful in determining the ____.
A) temperature of an object
B) distance to an object
C) chemical composition of an object
D) motion of an object across the observer's line of sight
E) motion of an object toward or away from the observer
A) temperature of an object
B) distance to an object
C) chemical composition of an object
D) motion of an object across the observer's line of sight
E) motion of an object toward or away from the observer

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
60
The formula 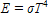 expresses the relationship between Kelvin temperature and the amount of radiated energy. If a star is three times the temperature of the Sun, how many times more intense is the energy radiated from an equal-sized area of its surface? (Answer with a number only.
expresses the relationship between Kelvin temperature and the amount of radiated energy. If a star is three times the temperature of the Sun, how many times more intense is the energy radiated from an equal-sized area of its surface? (Answer with a number only.
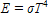 expresses the relationship between Kelvin temperature and the amount of radiated energy. If a star is three times the temperature of the Sun, how many times more intense is the energy radiated from an equal-sized area of its surface? (Answer with a number only.
expresses the relationship between Kelvin temperature and the amount of radiated energy. If a star is three times the temperature of the Sun, how many times more intense is the energy radiated from an equal-sized area of its surface? (Answer with a number only.
Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
61
Law enforcement officers use radar guns based on the Doppler effect to measure the speed of automobiles. Explain why the details of the Doppler effect make it desirable for the officers to park near the road in a place without curves.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
62
During the first year of its operation, only 20% of the projects scheduled for the Hubble Space Telescope involved taking images and pictures of celestial objects. The remaining 80% of the telescope's time was devoted to observing the spectrum of celestial objects.
Why do astronomers place such a high emphasis on looking at the spectrum of a celestial object rather than an image? Explain.
Why do astronomers place such a high emphasis on looking at the spectrum of a celestial object rather than an image? Explain.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
63
If 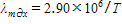 expresses the relationship between Kelvin temperature and the wavelength of maximum intensity in nanometers, what is the wavelength in nanometers of maximum intensity for a star with a surface temperature of 7,250K?
expresses the relationship between Kelvin temperature and the wavelength of maximum intensity in nanometers, what is the wavelength in nanometers of maximum intensity for a star with a surface temperature of 7,250K?
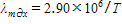 expresses the relationship between Kelvin temperature and the wavelength of maximum intensity in nanometers, what is the wavelength in nanometers of maximum intensity for a star with a surface temperature of 7,250K?
expresses the relationship between Kelvin temperature and the wavelength of maximum intensity in nanometers, what is the wavelength in nanometers of maximum intensity for a star with a surface temperature of 7,250K?
Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
64
What is the use of the Doppler effect able to tell you about the motion of an object? What is it unable to tell you?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
65
A hot blackbody emits a(n) _______________ spectrum.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
66
An object moving away from the observer will exhibit a(n) _______________ shift due to the Doppler effect.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
67
The Doppler formula states that the ratio of the velocity of a source to the speed of light is equal to the ratio of the change in wavelength caused by the Doppler shift to the source wavelength, or 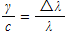
A spacecraft has yellow headlights which radiate 600nm photons, and is travelling toward a stationary observer. If the spacecraft is travelling at a velocity of 1% of the speed of light (0.01c), what will be the amount of the shift in wavelength observed (in nanometers)?
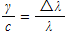
A spacecraft has yellow headlights which radiate 600nm photons, and is travelling toward a stationary observer. If the spacecraft is travelling at a velocity of 1% of the speed of light (0.01c), what will be the amount of the shift in wavelength observed (in nanometers)?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
68
What physical situation is necessary to create an absorption spectrum?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
69
A(n) _______________ spectrum is produced by a hot, excited gas.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
70
Name the three subatomic particles that make up an atom and state their electrical charges

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
71
Explain how the structure of the atom creates binding energy, and what limits there are on the amount of binding energy an electron can have.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
72
The _______________ effect changes the observed wavelength of an emitted photon if the source is moving toward or away from the observer.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
73
The NASA Curiosity rover exploring Mars carries a number of scientific instruments, including a spectrograph (a device for recording a spectrum), and a high intensity laser. On several occasions, Curiosity has fired its laser at the surface of a rock sample, while observing the process with a spectroscope. The laser is intense enough to vaporize the atoms on the surface of the sample into a hot, excited gas.
Why is this a useful technique for the rover to employ?
Why is this a useful technique for the rover to employ?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck


