Deck 11: Nuclear Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/143
Play
Full screen (f)
Deck 11: Nuclear Physics
1
Alpha, beta, and gamma particles are all deflected by a magnetic field.
False
2
The number of protons in a nucleus equals the number of neutrons.
False
3
The symbol indicating the number of neutrons in a nucleus is N .
True
4
In the case of alpha decay, the atomic number of the nucleus increases.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
5
The symbol indicating the mass number of a nucleus is A .

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
6
Helium always has two neutrons in its nucleus.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
7
When a nucleus in an excited state decays to the ground state, a beta particle is emitted.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
8
An alpha particle is another name for a helium nucleus.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
9
Two isotopes have the same mass number.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
10
The atomic number equals the number of protons in a nucleus.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
11
If one waits for a time equal to two half-lives for a certain radioactive material, all of it originally present will have decayed completely.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
12
A gamma ray is another name for an electron.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
13
The symbol indicating the number of nucleons in a nucleus is N .

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
14
When a neutron decays to a proton, a beta particle is emitted.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
15
The time interval during which a nucleus has a 50% probability of decaying is its half-life.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
16
When sulfur-35 ( Z = 16) decays to chlorine-35 ( Z = 17), an alpha particle is emitted.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
17
The symbol indicating the atomic number of a nucleus is A .

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
18
A beta particle is another name for a helium nucleus.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
19
The symbol indicating the number of protons in a nucleus is N .

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
20
When radium-223 ( Z = 88) decays to radon-119 ( Z = 86), an alpha particle is emitted.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
21
When uranium-238 ( Z = 92) is bombarded with a neutron, it can change into uranium-237.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
22
The half-life indicates how tightly the nucleons in a nucleus are bound together.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
23
Fusion of hydrogen nuclei is the origin of solar energy.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
24
Nuclear fusion is the source of energy in stars.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
25
The hydrogen in the universe was formed by the energy of the Big Bang.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
26
A Geiger counter registers a count rate of 8,000 counts per minute from a sample of a radioisotope. The count rate 24 minutes later is 1,000 counts per minute. The half-life of the radioisotope is 8 minutes.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
27
Gamma radiation is used to kill harmful bacteria in food.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
28
Magnetic confinement is used in controlled fusion experiments to control a plasma.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
29
Both nuclear reactors and atomic bombs use nuclear chain reactions.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
30
Carbon-14 dating can be used to find the age of material up to millions of years old.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
31
The mass of an atom of carbon-12 ( Z = 6) is less than the mass of 6 protons and 6 neutrons because of binding energy.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
32
Fusion is used to produce the largest nuclei known to exist.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
33
The determination of the composition of a material by bombarding it with neutrons and monitoring the emitted radiation is neutron activation analysis.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
34
A nuclear reactor could explode like an atomic bomb.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
35
Fission fragments are always identical.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
36
Atomic bombs and nuclear power plants both use nuclear fusion.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
37
The splitting of a large nucleus into two smaller nuclei upon absorbing a neutron is nuclear fusion.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
38
The combining of two nuclei to form a larger nucleus with the release of energy is nuclear fusion.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
39
The half-life of uranium-232 is 70 years. The time for 3/4 of a sample of uranium-232 to decay is 105 years.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
40
The product of a radioactive decay is called the "daughter."

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
41
The number of protons in a nucleus is the
A) mass number.
B) neutron number.
C) atomic number.
D) isotope number.
E) none of the above
A) mass number.
B) neutron number.
C) atomic number.
D) isotope number.
E) none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
42
How can an electron be ejected from a nucleus in beta decay if it wasn't in the nucleus to begin with?
A) The electron doesn't come from the nucleus-it comes from an orbit circling the nucleus.
B) A neutron in the nucleus turns into a proton, electron, and a neutrino.
C) A proton in the nucleus turns into a neutron, electron, and a neutrino.
D) none of the above
A) The electron doesn't come from the nucleus-it comes from an orbit circling the nucleus.
B) A neutron in the nucleus turns into a proton, electron, and a neutrino.
C) A proton in the nucleus turns into a neutron, electron, and a neutrino.
D) none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
43
The expression(s) indicating the atomic number of a nucleus is
A) A.
B) N.
C) Z.
D) Z + N.
E) A - Z.
A) A.
B) N.
C) Z.
D) Z + N.
E) A - Z.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
44
An alpha particle is like 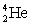 with 2 protons and 2 neutrons, whereas a beta particle is equivalent to hydrogen.
with 2 protons and 2 neutrons, whereas a beta particle is equivalent to hydrogen.
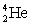 with 2 protons and 2 neutrons, whereas a beta particle is equivalent to hydrogen.
with 2 protons and 2 neutrons, whereas a beta particle is equivalent to hydrogen.
Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
45
If someone tells you they have a sample of niobium-90, can you tell how many neutrons are in the nucleus?
A) Yes, there are 41 neutrons.
B) Yes, there are 90 neutrons.
C) Yes, there are 49 neutrons.
D) No, more information must be given.
E) No, there is no such thing as "niobium."
A) Yes, there are 41 neutrons.
B) Yes, there are 90 neutrons.
C) Yes, there are 49 neutrons.
D) No, more information must be given.
E) No, there is no such thing as "niobium."

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
46
Low levels of ionizing radiation
A) are dangerous to the human body.
B) can be tolerated by the human body.
C) are beneficial to the human body.
D) none of the above
A) are dangerous to the human body.
B) can be tolerated by the human body.
C) are beneficial to the human body.
D) none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
47
What is 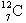 ?
?
A) There is no such thing.
B) an isotope of carbon with 7 neutrons and 12 protons
C) an isotope of carbon with 7 neutrons and 5 protons
D) an isotope of carbon with 5 neutrons and 7 protons
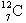 ?
?A) There is no such thing.
B) an isotope of carbon with 7 neutrons and 12 protons
C) an isotope of carbon with 7 neutrons and 5 protons
D) an isotope of carbon with 5 neutrons and 7 protons

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
48
Two isotopes have the same
A) mass number.
B) neutron number.
C) atomic number.
D) quantum number.
E) none of the above
A) mass number.
B) neutron number.
C) atomic number.
D) quantum number.
E) none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
49
Einstein's equation E = mc 2 states that when a particle loses energy, the result is an increase in mass of the particle.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
50
After three half-lives, 3/4 of the nuclei in a sample of a radioisotope have decayed

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
51
The energy of nuclear radiation as it is absorbed mostly goes to heat the material.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
52
What does the 235 represent in uranium-235?
A) the mass number
B) the atomic number
C) the neutron number
D) none of the above
A) the mass number
B) the atomic number
C) the neutron number
D) none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
53
The expression(s) indicating the number of protons in a nucleus is
A) A.
B) N.
C) Z.
D) Z + N.
E) A - Z.
A) A.
B) N.
C) Z.
D) Z + N.
E) A - Z.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
54
An alpha particle is another name for a(n)
A) electron.
B) proton.
C) neutron.
D) helium nucleus.
E) photon.
A) electron.
B) proton.
C) neutron.
D) helium nucleus.
E) photon.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
55
What's an isotope?
A) a radioactive material
B) an atom of an element with a different number of neutrons in the nucleus
C) an electrically charged atom
D) none of the above
A) a radioactive material
B) an atom of an element with a different number of neutrons in the nucleus
C) an electrically charged atom
D) none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
56
A beta particle is another name for a(n)
A) electron.
B) proton.
C) neutron.
D) helium nucleus.
E) photon.
A) electron.
B) proton.
C) neutron.
D) helium nucleus.
E) photon.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
57
How big is the nucleus of an atom?
A) about 1% of the volume of the whole atom
B) about a millionth of the volume of the whole atom
C) about a billionth of the volume of the whole atom
D) about a trillionth of the volume of the whole atom
A) about 1% of the volume of the whole atom
B) about a millionth of the volume of the whole atom
C) about a billionth of the volume of the whole atom
D) about a trillionth of the volume of the whole atom

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
58
If a substance is radioactive it is definitely
A) dangerous.
B) emitting alpha particles.
C) glowing.
D) useful.
E) all of the above.
F) none of the above.
A) dangerous.
B) emitting alpha particles.
C) glowing.
D) useful.
E) all of the above.
F) none of the above.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
59
What holds the nucleus together?
A) electrical forces between the protons
B) alpha particles
C) beta particles
D) strong forces
A) electrical forces between the protons
B) alpha particles
C) beta particles
D) strong forces

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
60
A gamma ray is another name for a(n)
A) electron.
B) proton.
C) neutron.
D) helium nucleus.
E) photon.
A) electron.
B) proton.
C) neutron.
D) helium nucleus.
E) photon.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
61
What's a decay chain?
A) a nuclear chain reaction
B) one radioisotope decaying into another, which decays into another, and so on
C) the triggering of multiple fissions by a single neutron
D) none of the above
A) a nuclear chain reaction
B) one radioisotope decaying into another, which decays into another, and so on
C) the triggering of multiple fissions by a single neutron
D) none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
62
A Geiger counter registers a count rate of 8,000 counts per minute from a sample of a radioisotope. The count rate 24 minutes later is 1,000 counts per minute. The half-life of the radioisotope is
A) 3 minutes.
B) 4 minutes.
C) 6 minutes.
D) 8 minutes.
E) 12 minutes.
A) 3 minutes.
B) 4 minutes.
C) 6 minutes.
D) 8 minutes.
E) 12 minutes.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
63
Why is there a radioactive source in a smoke detector?
A) It makes the smoke particles radioactive.
B) It ionizes the air.
C) It powers the alarm when the battery wears out.
D) none of the above
A) It makes the smoke particles radioactive.
B) It ionizes the air.
C) It powers the alarm when the battery wears out.
D) none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
64
Carbon-14 dating can be used to find the age of material up to ____________ of years old.
A) millions
B) billions
C) hundreds of thousands
D) tens of thousands
A) millions
B) billions
C) hundreds of thousands
D) tens of thousands

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
65
The combining of two nuclei to form a larger nucleus with the release of energy is
A) nuclear fission.
B) nuclear fusion.
C) neutron activation.
D) nuclear emission.
E) beta decay.
A) nuclear fission.
B) nuclear fusion.
C) neutron activation.
D) nuclear emission.
E) beta decay.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
66
How tightly the nucleons in a nucleus are bound together is indicated by the nucleus's
A) atomic number.
B) mass number.
C) neutron number.
D) half-life.
E) binding energy.
A) atomic number.
B) mass number.
C) neutron number.
D) half-life.
E) binding energy.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
67
What does the moderator do in a nuclear reactor?
A) It is a safety feature that limits the nuclear reaction.
B) It absorbs neutrons.
C) It slows down neutrons.
D) none of the above
A) It is a safety feature that limits the nuclear reaction.
B) It absorbs neutrons.
C) It slows down neutrons.
D) none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
68
What do "kiloton" and "megaton" refer to?
A) explosive yields of nuclear weapons
B) kiloton means 1000 tons of TNT
C) megaton means one million tons of TNT
D) all of the above
A) explosive yields of nuclear weapons
B) kiloton means 1000 tons of TNT
C) megaton means one million tons of TNT
D) all of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
69
The hydrogen in the universe was formed by
A) the energy of the Big Bang.
B) nuclear fusion reactions in stars.
C) radioactive decay of heavy elements.
D) none of the above.
A) the energy of the Big Bang.
B) nuclear fusion reactions in stars.
C) radioactive decay of heavy elements.
D) none of the above.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
70
When radium-223 ( Z = 88) decays to radon-119 ( Z = 86), the other particle emitted is
A) an alpha particle.
B) a beta particle.
C) a gamma ray.
D) an x-ray.
E) none of the above.
A) an alpha particle.
B) a beta particle.
C) a gamma ray.
D) an x-ray.
E) none of the above.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following is not a method of producing nuclear fusion?
A) heating a magnetically confined plasma
B) forcing nuclei together using lasers
C) cooling nuclei to very cold temperatures
D) forcing nuclei together with a nuclear fission explosion
A) heating a magnetically confined plasma
B) forcing nuclei together using lasers
C) cooling nuclei to very cold temperatures
D) forcing nuclei together with a nuclear fission explosion

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
72
Why is nuclear reactor waste radioactive?
A) Radiation from the reactor makes everything in or near it radioactive.
B) Used fuel rods contain fission fragments which are radioactive.
C) Spent fuel rods pick up contamination from the reactor.
D) all of the above
A) Radiation from the reactor makes everything in or near it radioactive.
B) Used fuel rods contain fission fragments which are radioactive.
C) Spent fuel rods pick up contamination from the reactor.
D) all of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
73
The splitting of a large nucleus into two smaller nuclei upon absorbing a neutron is
A) nuclear fission.
B) nuclear fusion.
C) neutron activation.
D) nuclear emission.
E) beta decay.
A) nuclear fission.
B) nuclear fusion.
C) neutron activation.
D) nuclear emission.
E) beta decay.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following is not the usual outcome of nuclear fission?
A) The binding energy per nucleon of the fission fragments is smaller than that of the original nucleus.
B) Energy is released.
C) Neutrons are released.
D) The fission fragments are radioactive.
A) The binding energy per nucleon of the fission fragments is smaller than that of the original nucleus.
B) Energy is released.
C) Neutrons are released.
D) The fission fragments are radioactive.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
75
When a neutron decays to create a proton, another particle emitted is
A) an alpha particle.
B) a beta particle.
C) a gamma ray.
D) an x-ray.
E) none of the above.
A) an alpha particle.
B) a beta particle.
C) a gamma ray.
D) an x-ray.
E) none of the above.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
76
The time interval during which a nucleus has a 50% probability of decaying is its
A) half-life.
B) decay time.
C) nuclei activation time.
D) binding energy time.
E) none of the above.
A) half-life.
B) decay time.
C) nuclei activation time.
D) binding energy time.
E) none of the above.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
77
When sulfur-35 ( Z = 16) decays to chlorine-35 ( Z = 17), a particle emitted is
A) an alpha particle.
B) a beta particle.
C) a gamma ray.
D) an x-ray.
E) none of the above.
A) an alpha particle.
B) a beta particle.
C) a gamma ray.
D) an x-ray.
E) none of the above.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
78
When a nucleus in an excited state decays to the ground state, the particle emitted is
A) an alpha particle.
B) a beta particle.
C) a gamma ray.
D) an x-ray.
E) none of the above.
A) an alpha particle.
B) a beta particle.
C) a gamma ray.
D) an x-ray.
E) none of the above.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
79
What do you do to the control rods of a reactor in order to shut it down?
A) push them into the core
B) pull them out of the core
C) nothing-it's the cooling water that shuts down the reactor
D) none of the above
A) push them into the core
B) pull them out of the core
C) nothing-it's the cooling water that shuts down the reactor
D) none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
80
The half-life of uranium-232 is 70 years. The time for 3/4 of a sample of uranium-232 to decay is
A) 35 years.
B) 70 years.
C) 125 years.
D) 140 years.
E) none of the above.
A) 35 years.
B) 70 years.
C) 125 years.
D) 140 years.
E) none of the above.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck


