Deck 4: Chemical Reactions in Solution
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/107
Play
Full screen (f)
Deck 4: Chemical Reactions in Solution
1
Using solubility guidelines, which of the following ionic compounds is not properly labeled with respect to solubility in water?
A) NiCl2 is soluble.
B) Ag2S is insoluble.
C) Cs3PO4 is insoluble.
D) SrCO3 is insoluble.
E) (NH4)2SO4 is soluble.
A) NiCl2 is soluble.
B) Ag2S is insoluble.
C) Cs3PO4 is insoluble.
D) SrCO3 is insoluble.
E) (NH4)2SO4 is soluble.
Cs3PO4 is insoluble.
2
Which compound listed below is not soluble in water?
A) KCl
B) (NH4)2CO3
C) AgNO3
D) PbSO4
E) none of these
A) KCl
B) (NH4)2CO3
C) AgNO3
D) PbSO4
E) none of these
PbSO4
3
Which of the following ionic compounds are expected to be soluble in water?
I. PbCl2
II. Cu(NO3)2
III. BaSO4
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. PbCl2
II. Cu(NO3)2
III. BaSO4
A) I only
B) II only
C) III only
D) I and II
E) All of these
II only
4
Choose the correct net ionic equation for the reaction in water of CaCl2 and Na3PO4.
A) 3 CaCl2 (aq) + 2 Na3PO4 (aq)→ Ca3(PO4)2 (s) + 6 NaCl (aq)
B) Ca2+ (aq) + PO43 - (aq)→ Ca(PO4) (s)
C) 3 Ca2+ (aq) + 2 PO43 - (aq)→ Ca3(PO4)2 (s)
D) Cl - (aq) + Na+ (aq)→ NaCl (aq)
E) none of these
A) 3 CaCl2 (aq) + 2 Na3PO4 (aq)→ Ca3(PO4)2 (s) + 6 NaCl (aq)
B) Ca2+ (aq) + PO43 - (aq)→ Ca(PO4) (s)
C) 3 Ca2+ (aq) + 2 PO43 - (aq)→ Ca3(PO4)2 (s)
D) Cl - (aq) + Na+ (aq)→ NaCl (aq)
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
5
Exhibit 4-1 Consider the precipitation reaction below to answer the following problem(s):
AgNO3 (aq) + K3PO4 (aq)→??
Refer to Exhibit 4-1. What two products are formed in this precipitation reaction?
A) Ag3PO4 and KNO3
B) AgPO4 and K3NO3
C) Ag3N and K3P and O2
D) Ag3P and K3N and O2
E) AgK3 and NO3PO4
AgNO3 (aq) + K3PO4 (aq)→??
Refer to Exhibit 4-1. What two products are formed in this precipitation reaction?
A) Ag3PO4 and KNO3
B) AgPO4 and K3NO3
C) Ag3N and K3P and O2
D) Ag3P and K3N and O2
E) AgK3 and NO3PO4

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
6
A chemist places AgNO3 in one flask and NaBr in another. Water is added to both flasks and the mixture in the first flask is added to the second. Which choice below describes correctly the results of this experiment?
A) Both of the compounds in the flasks dissolve when water is added and AgBr precipitates when the contents of the flasks are mixed.
B) Both of the compounds in the flasks dissolve when water is added and NaNO3 precipitates when the contents of the flasks are mixed.
C) Both of the compounds in the flasks dissolve when water is added and there is no precipitate when the contents of the flasks are mixed.
D) The AgNO3 does not dissolve in water, but the NaBr does dissolve. There is no change upon mixing the contents of the flasks.
E) none of these
A) Both of the compounds in the flasks dissolve when water is added and AgBr precipitates when the contents of the flasks are mixed.
B) Both of the compounds in the flasks dissolve when water is added and NaNO3 precipitates when the contents of the flasks are mixed.
C) Both of the compounds in the flasks dissolve when water is added and there is no precipitate when the contents of the flasks are mixed.
D) The AgNO3 does not dissolve in water, but the NaBr does dissolve. There is no change upon mixing the contents of the flasks.
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
7
Choose the correct net ionic equation for the reaction of HCl and NaOH taking place in H2O.
A) 2 H+ (aq) + OH - (aq)→ H3O+ (aq)
B) H + (aq) + OH - (aq) H₂ O (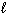 )
)
C) 2 Na+ (aq) + 2 Cl - (aq)→ 2 NaCl (aq)
D) 2 H + (aq) + 2 Cl - (aq) + 2 Na + (aq) + 2 OH - (aq) 2 H₂ O (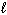 ) + 2 NaCl (aq)
) + 2 NaCl (aq)
E) none of these
A) 2 H+ (aq) + OH - (aq)→ H3O+ (aq)
B) H + (aq) + OH - (aq) H₂ O (
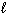 )
)C) 2 Na+ (aq) + 2 Cl - (aq)→ 2 NaCl (aq)
D) 2 H + (aq) + 2 Cl - (aq) + 2 Na + (aq) + 2 OH - (aq) 2 H₂ O (
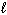 ) + 2 NaCl (aq)
) + 2 NaCl (aq)E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
8
Which compound listed below is soluble in water?
A) BaSO4
B) AgCl
C) MgCO3
D) Na2CO3
E) none of these
A) BaSO4
B) AgCl
C) MgCO3
D) Na2CO3
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
9
What products are expected and what are the coefficients for these products in a precipitation reaction when the following aqueous solutions are mixed?
3 AgNO3 (aq) + Cs3PO4 (aq)→??
A) AgPO4 (s) + Cs3NO3 (aq)
B) Ag2PO4 (s) + 3 Cs(NO3)2
C) Ag3PO4 (s) + 3 CsNO3 (aq)
D) AgPO4 (s) + 3 CsNO3 (aq)
E) 2 Ag3N (s) + 2 Cs3P + 11 O2
3 AgNO3 (aq) + Cs3PO4 (aq)→??
A) AgPO4 (s) + Cs3NO3 (aq)
B) Ag2PO4 (s) + 3 Cs(NO3)2
C) Ag3PO4 (s) + 3 CsNO3 (aq)
D) AgPO4 (s) + 3 CsNO3 (aq)
E) 2 Ag3N (s) + 2 Cs3P + 11 O2

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
10
Which compound listed below is not soluble in water?
A) BaCO3
B) BaCl2
C) Na2CO3
D) Na2SO4
E) none of these
A) BaCO3
B) BaCl2
C) Na2CO3
D) Na2SO4
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
11
Which compound listed below is soluble in water?
A) BaCl2
B) AgBr
C) PbSO4
D) Be(OH)2
E) none of these
A) BaCl2
B) AgBr
C) PbSO4
D) Be(OH)2
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
12
Exhibit 4-1 Consider the precipitation reaction below to answer the following problem(s):
AgNO3 (aq) + K3PO4 (aq)→??
Refer to Exhibit 4-1. What is the correct formula for the compound that would precipitate as an insoluble solid from the precipitation reaction above?
A) Ag3PO4
B) KNO3
C) AgPO4
D) Ag3N
E) AgK3
AgNO3 (aq) + K3PO4 (aq)→??
Refer to Exhibit 4-1. What is the correct formula for the compound that would precipitate as an insoluble solid from the precipitation reaction above?
A) Ag3PO4
B) KNO3
C) AgPO4
D) Ag3N
E) AgK3

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
13
Which compound listed below is not soluble in water?
A) NH4Cl
B) CaCl2
C) NaOH
D) PbCl2
E) none of these
A) NH4Cl
B) CaCl2
C) NaOH
D) PbCl2
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
14
Which compound listed below is soluble in water?
A) PbCl2
B) AgNO3
C) Fe(OH)3
D) CaCO3
E) none of these
A) PbCl2
B) AgNO3
C) Fe(OH)3
D) CaCO3
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
15
A chemist places Ba(NO3)2 in one flask and Na2SO4 in another. Water is added to both flasks and the mixture in the first flask is added to the second. Which choice below describes correctly the results of this experiment?
A) Both of the compounds in the flasks dissolve when water is added and NaNO3 precipitates when the contents of the flasks are mixed.
B) Both of the compounds in the flasks dissolve when water is added and BaSO4 precipitates when the contents of the flasks are mixed.
C) Both of the compounds in the flasks dissolve when water is added and there is no precipitate when the contents of the flasks are mixed.
D) The Ba(NO3)2 does not dissolve in water, but the Na2SO4 does dissolve. There is no change upon mixing the contents of the flasks.
E) none of these
A) Both of the compounds in the flasks dissolve when water is added and NaNO3 precipitates when the contents of the flasks are mixed.
B) Both of the compounds in the flasks dissolve when water is added and BaSO4 precipitates when the contents of the flasks are mixed.
C) Both of the compounds in the flasks dissolve when water is added and there is no precipitate when the contents of the flasks are mixed.
D) The Ba(NO3)2 does not dissolve in water, but the Na2SO4 does dissolve. There is no change upon mixing the contents of the flasks.
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
16
Which compound listed below is not soluble in water?
A) Na2CO3
B) CuCl2
C) Fe(NO3)3
D) BaSO4
E) NH4OH
A) Na2CO3
B) CuCl2
C) Fe(NO3)3
D) BaSO4
E) NH4OH

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
17
Choose the correct net ionic equation for the reaction in water of AgNO3 and BaCl2.
A) 2 AgNO3 (aq) + BaCl2 (aq)→ 2 AgCl (s) + Ba(NO3)2 (aq)
B) Ag2+ (aq) + 2 Cl - (aq)→ AgCl2 (s)
C) Ag+ (aq) + Cl - (aq)→ AgCl (s)
D) Ba2+ (aq) + 2 NO3 - (aq)→ Ba(NO3)2 (aq)
E) none of these
A) 2 AgNO3 (aq) + BaCl2 (aq)→ 2 AgCl (s) + Ba(NO3)2 (aq)
B) Ag2+ (aq) + 2 Cl - (aq)→ AgCl2 (s)
C) Ag+ (aq) + Cl - (aq)→ AgCl (s)
D) Ba2+ (aq) + 2 NO3 - (aq)→ Ba(NO3)2 (aq)
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
18
A chemist places CaCO3 in one flask and KBr in another. Water is added to both flasks and the mixture in the first flask is added to the second. Which choice below describes correctly the results of this experiment?
A) Both of the compounds in the flasks dissolve when water is added and CaBr2 precipitates when the contents of the flasks are mixed.
B) Both of the compounds in the flasks dissolve when water is added and K2CO3 precipitates when the contents of the flasks are mixed.
C) Both of the compounds in the flasks dissolve when water is added and there is no precipitate when the contents of the flasks are mixed.
D) The CaCO3 does not dissolve in water, but the KBr does dissolve. There is no change upon mixing the contents of the two flasks.
E) none of these
A) Both of the compounds in the flasks dissolve when water is added and CaBr2 precipitates when the contents of the flasks are mixed.
B) Both of the compounds in the flasks dissolve when water is added and K2CO3 precipitates when the contents of the flasks are mixed.
C) Both of the compounds in the flasks dissolve when water is added and there is no precipitate when the contents of the flasks are mixed.
D) The CaCO3 does not dissolve in water, but the KBr does dissolve. There is no change upon mixing the contents of the two flasks.
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following ionic compounds is considered insoluble in water?
A) (NH4)3PO4
B) Mg(OH)2
C) Na2SO4
D) Pb(NO3)2
E) Mg(C2H3O2)2
A) (NH4)3PO4
B) Mg(OH)2
C) Na2SO4
D) Pb(NO3)2
E) Mg(C2H3O2)2

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
20
A chemist places (NH4)2SO4 in one flask and NaI in another. Water is added to both flasks and the mixture in the first flask is added to the second. Which choice below describes correctly the results of this experiment?
A) Both of the compounds in the flasks dissolve when water is added and NH4I precipitates when the contents of the flasks are mixed.
B) Both of the compounds in the flasks dissolve when water is added and Na2SO4 precipitates when the contents of the flasks are mixed.
C) Both of the compounds in the flasks dissolve when water is added and there is no precipitate when the contents of the flasks are mixed.
D) The (NH4)2SO4 does not dissolve in water, but the NaI does dissolve. There is no change upon mixing the contents of flasks
E) none of these
A) Both of the compounds in the flasks dissolve when water is added and NH4I precipitates when the contents of the flasks are mixed.
B) Both of the compounds in the flasks dissolve when water is added and Na2SO4 precipitates when the contents of the flasks are mixed.
C) Both of the compounds in the flasks dissolve when water is added and there is no precipitate when the contents of the flasks are mixed.
D) The (NH4)2SO4 does not dissolve in water, but the NaI does dissolve. There is no change upon mixing the contents of flasks
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
21
What mass of NaOH is needed to make 450 mL of a 2.0 molar solution?
A) 18 g
B) 42 g
C) 36 g
D) 24 g
E) none of these
A) 18 g
B) 42 g
C) 36 g
D) 24 g
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
22
What is the definition for Molarity ?
A) Grams of solute per moles of solute
B) Moles of solute per grams of solution
C) Moles of solute per Liter of solvent
D) Grams of solute per Liter of solution
E) Moles of solute per Liter of solution
A) Grams of solute per moles of solute
B) Moles of solute per grams of solution
C) Moles of solute per Liter of solvent
D) Grams of solute per Liter of solution
E) Moles of solute per Liter of solution

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
23
Exhibit 4-2 Consider mixing an aqueous solution of FeCl3 with an aqueous solution of KOH:
FeCl3 (aq) + KOH (aq)→??
Refer to Exhibit 4-2. What is the net ionic equation for this precipitation reaction?
A) Fe3+ (aq) + 3 OH - (aq)→ Fe(OH)3 (s)
B) Fe3+ (aq) + 3 Cl - (aq)→ FeCl3 (s)
C) Fe3+ (aq) + 3 OH - (aq)→ FeOH3 (s)
D) 3 K+ (aq) + 3 Cl - (aq)→ 3 KCl (s)
E) K+ (aq) + Cl3 (aq)→ KCl3 (s)
FeCl3 (aq) + KOH (aq)→??
Refer to Exhibit 4-2. What is the net ionic equation for this precipitation reaction?
A) Fe3+ (aq) + 3 OH - (aq)→ Fe(OH)3 (s)
B) Fe3+ (aq) + 3 Cl - (aq)→ FeCl3 (s)
C) Fe3+ (aq) + 3 OH - (aq)→ FeOH3 (s)
D) 3 K+ (aq) + 3 Cl - (aq)→ 3 KCl (s)
E) K+ (aq) + Cl3 (aq)→ KCl3 (s)

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
24
Which equation is the net ionic equation for the reaction in water of BeCl2 and K2CO3?
A) BeCl2 (aq) + K2CO3 (aq)→ BeCO3 (s) + 2 KCl (aq)
B) BeCl2 (s)→ Be2+ (aq) + 2 Cl - (aq)
C) K+ (aq) + Cl - (aq)→ KCl (s)
D) Be2+ (aq) + CO32 - (aq)→ BeCO3 (s)
E) none of these
A) BeCl2 (aq) + K2CO3 (aq)→ BeCO3 (s) + 2 KCl (aq)
B) BeCl2 (s)→ Be2+ (aq) + 2 Cl - (aq)
C) K+ (aq) + Cl - (aq)→ KCl (s)
D) Be2+ (aq) + CO32 - (aq)→ BeCO3 (s)
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
25
What is the molarity of a solution if 0.50 mole of CaCl2 is dissolved to make 2.00 L of solution?
A) 1.0 molar
B) 2.0 molar
C) 0.50 molar
D) 0.25 molar
E) none of these
A) 1.0 molar
B) 2.0 molar
C) 0.50 molar
D) 0.25 molar
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
26
Which ions are considered spectators in the reaction between aqueous solutions of (NH4)3PO4 and CaCl2?
A) 3 Ca2+ (aq) and 2 PO43 - (aq)
B) 6 N3 - , 24 H+ (aq) and 6 Cl - (aq)
C) 6 NH4+ (aq) and 6 Cl - (aq)
D) 3 Ca2+ (aq) and 6 Cl - (aq)
E) 6 NH4+ (aq) and 2 PO43 - (aq)
A) 3 Ca2+ (aq) and 2 PO43 - (aq)
B) 6 N3 - , 24 H+ (aq) and 6 Cl - (aq)
C) 6 NH4+ (aq) and 6 Cl - (aq)
D) 3 Ca2+ (aq) and 6 Cl - (aq)
E) 6 NH4+ (aq) and 2 PO43 - (aq)

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
27
Exhibit 4-2 Consider mixing an aqueous solution of FeCl3 with an aqueous solution of KOH:
FeCl3 (aq) + KOH (aq)→??
Refer to Exhibit 4-2. What is the overall equation for the precipitation reaction that ensues?
A) FeCl3 (aq) + KOH (aq)→ FeOH (s) + KCl3 (aq)
B) FeCl3 (aq) + 3 KOH (aq)→ FeOH3 (s) + 3 KCl3 (aq)
C) FeCl3 (aq) + 3 KOH (aq)→ Fe(OH)3 (s) + 3 KCl (aq)
D) FeCl3 (aq) + KOH (aq)→ FeK (s) + Cl3OH (aq)
E) FeCl3 (aq) + KOH (aq)→ FeCl3 (aq) + KOH (aq)
FeCl3 (aq) + KOH (aq)→??
Refer to Exhibit 4-2. What is the overall equation for the precipitation reaction that ensues?
A) FeCl3 (aq) + KOH (aq)→ FeOH (s) + KCl3 (aq)
B) FeCl3 (aq) + 3 KOH (aq)→ FeOH3 (s) + 3 KCl3 (aq)
C) FeCl3 (aq) + 3 KOH (aq)→ Fe(OH)3 (s) + 3 KCl (aq)
D) FeCl3 (aq) + KOH (aq)→ FeK (s) + Cl3OH (aq)
E) FeCl3 (aq) + KOH (aq)→ FeCl3 (aq) + KOH (aq)

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
28
What is the balanced net ionic equation for the result when the following two aqueous solutions are mixed?
CaCl2 (aq) and Cs3PO4 (aq)
A) Ca2+ (aq) + 2 Cl - (aq)→ CaCl2 (s)
B) Ca2+ (aq) + PO43 - (aq)→ CaPO4 (s)
C) Cs+ (aq) + Cl - (aq)→ CsCl (s)
D) 2 Ca2+ (aq) + 3 PO43 - (aq)→ Ca2(PO4)3 (s)
E) 3 Ca2+ (aq) + 2 PO43 - (aq)→ Ca3(PO4)2 (s)
CaCl2 (aq) and Cs3PO4 (aq)
A) Ca2+ (aq) + 2 Cl - (aq)→ CaCl2 (s)
B) Ca2+ (aq) + PO43 - (aq)→ CaPO4 (s)
C) Cs+ (aq) + Cl - (aq)→ CsCl (s)
D) 2 Ca2+ (aq) + 3 PO43 - (aq)→ Ca2(PO4)3 (s)
E) 3 Ca2+ (aq) + 2 PO43 - (aq)→ Ca3(PO4)2 (s)

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
29
What mass of KOH is needed to prepare 0.50 L of a 0.100 molar solution?
A) 0.22 g
B) 4.3 g
C) 1.1 g
D) 2.8 g
E) none of these
A) 0.22 g
B) 4.3 g
C) 1.1 g
D) 2.8 g
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
30
Which equation shown below represents the total ionic equation for the reaction between aqueous solutions of (NH4)3PO4 and CaCl2?
A) 6 NH3 (g) + 6 H+ (aq) + 2 PO43 - (aq) + 3 Ca2+ (aq) + 3 Cl22 - (aq)→ 3 Ca2+ (aq) + 2 PO43 - (aq) + 3 (NH4)2Cl2 (s)
B) 6 N3 - (aq) + 24 H+ (aq) + 2 P3 - (aq) + 8 O2 - (aq) + 3 Ca2+ (aq) + 6 Cl - (aq)→ Ca3(PO4)2 (s) + 6 N3 - (aq) + 24 H+ (aq) + 6 Cl - (aq)
C) 3 NH4+ (aq) + PO43 - (aq) + Ca2+ (aq) + 2 Cl - (aq)→ CaPO4 (s) + 3 NH4+ (aq) + 2 Cl - (aq)
D) 6 NH4+ (aq) + 2 PO43 - (aq) + 3 Ca2+ (aq) + 6 Cl - (aq)→ 3 Ca2+ (aq) + 2 PO43 - (aq) + 6 NH4Cl (s)
E) 6 NH4+ (aq) + 2 PO43 - (aq) + 3 Ca2+ (aq) + 6 Cl - (aq)→ Ca3(PO4)2 (s) + 6 NH4+ (aq) + 6 Cl - (aq)
A) 6 NH3 (g) + 6 H+ (aq) + 2 PO43 - (aq) + 3 Ca2+ (aq) + 3 Cl22 - (aq)→ 3 Ca2+ (aq) + 2 PO43 - (aq) + 3 (NH4)2Cl2 (s)
B) 6 N3 - (aq) + 24 H+ (aq) + 2 P3 - (aq) + 8 O2 - (aq) + 3 Ca2+ (aq) + 6 Cl - (aq)→ Ca3(PO4)2 (s) + 6 N3 - (aq) + 24 H+ (aq) + 6 Cl - (aq)
C) 3 NH4+ (aq) + PO43 - (aq) + Ca2+ (aq) + 2 Cl - (aq)→ CaPO4 (s) + 3 NH4+ (aq) + 2 Cl - (aq)
D) 6 NH4+ (aq) + 2 PO43 - (aq) + 3 Ca2+ (aq) + 6 Cl - (aq)→ 3 Ca2+ (aq) + 2 PO43 - (aq) + 6 NH4Cl (s)
E) 6 NH4+ (aq) + 2 PO43 - (aq) + 3 Ca2+ (aq) + 6 Cl - (aq)→ Ca3(PO4)2 (s) + 6 NH4+ (aq) + 6 Cl - (aq)

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
31
Which equation is the net ionic equation for the reaction in water of Pb(NO3)2 and CsI?
A) Pb(NO3)2 (aq) + CsI (aq)→ PbI2 (s) + 2 CsNO3 (aq)
B) Pb(NO3)2 (s)→ Pb2+ (aq) + 2 NO3 - (aq)
C) Cs+ (aq) + NO3 - (aq)→ CsNO3 (s)
D) Pb2+ (aq) + 2 I - (aq)→ PbI2 (s)
E) none of these
A) Pb(NO3)2 (aq) + CsI (aq)→ PbI2 (s) + 2 CsNO3 (aq)
B) Pb(NO3)2 (s)→ Pb2+ (aq) + 2 NO3 - (aq)
C) Cs+ (aq) + NO3 - (aq)→ CsNO3 (s)
D) Pb2+ (aq) + 2 I - (aq)→ PbI2 (s)
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
32
What is the molarity of a solution containing 17.0 grams of NH3 in 1.20 liters of solution?
A) 0.0706 M
B) 0.833 M
C) 1.20 M
D) 14.2 M
E) None of these
A) 0.0706 M
B) 0.833 M
C) 1.20 M
D) 14.2 M
E) None of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
33
What is the molarity of an aqueous solution prepared by dissolving 25.0 grams of Na2SO4 in enough water to form 500 mL of solution?
Molar Mass(Na2SO4) = 142.0 g/mol
A) 3.52×10 - 4 M Na2SO4
B) 0.0500 M Na2SO4
C) 0.176 M Na2SO4
D) 0.352 M Na2SO4
E) 500 M Na2SO4
Molar Mass(Na2SO4) = 142.0 g/mol
A) 3.52×10 - 4 M Na2SO4
B) 0.0500 M Na2SO4
C) 0.176 M Na2SO4
D) 0.352 M Na2SO4
E) 500 M Na2SO4

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
34
What is the molarity of a solution prepared by dissolving 3.4 g of NH3 in 500 mL of solution?
A) 4.0×10 - 4 M
B) 6.8×10 - 3 M
C) 4.0×10 - 1 M
D) 6.8 M
E) none of these
A) 4.0×10 - 4 M
B) 6.8×10 - 3 M
C) 4.0×10 - 1 M
D) 6.8 M
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
35
Which equation is the net ionic equation for the reaction in water of BaCl2 and Na2SO4?
A) BaCl2 (aq) + Na2SO4 (aq)→ BaSO4 (s) + 2 NaCl (aq)
B) BaCl2 (s)→ Ba2+ (aq) + 2 Cl - (aq)
C) Ba2+ (aq) + SO42 - (aq)→ BaSO4 (s)
D) Na+ (aq) + Cl - (aq)→ NaCl (s)
E) none of these
A) BaCl2 (aq) + Na2SO4 (aq)→ BaSO4 (s) + 2 NaCl (aq)
B) BaCl2 (s)→ Ba2+ (aq) + 2 Cl - (aq)
C) Ba2+ (aq) + SO42 - (aq)→ BaSO4 (s)
D) Na+ (aq) + Cl - (aq)→ NaCl (s)
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
36
Exhibit 4-2 Consider mixing an aqueous solution of FeCl3 with an aqueous solution of KOH:
FeCl3 (aq) + KOH (aq)→??
Refer to Exhibit 4-2. What spectator ions are present in this precipitation reaction?
A) Fe+ (aq) and 3 Cl - (aq)
B) K+ (aq) and OH - (aq)
C) Fe3+ (aq) and 3 OH - (aq)
D) 3 K+ and 3 Cl - (aq)
E) K3+ (aq) and Cl3 - (aq)
FeCl3 (aq) + KOH (aq)→??
Refer to Exhibit 4-2. What spectator ions are present in this precipitation reaction?
A) Fe+ (aq) and 3 Cl - (aq)
B) K+ (aq) and OH - (aq)
C) Fe3+ (aq) and 3 OH - (aq)
D) 3 K+ and 3 Cl - (aq)
E) K3+ (aq) and Cl3 - (aq)

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
37
What is the molarity of a solution containing 29.2 grams of NaCl in 0.60 liters of solution?
MM(NaCl) = 58.4 g/mol
A) 0.300 M
B) 0.833 M
C) 3.33 M
D) 48.7 M
E) 1.20 M
MM(NaCl) = 58.4 g/mol
A) 0.300 M
B) 0.833 M
C) 3.33 M
D) 48.7 M
E) 1.20 M

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
38
What mass of I2 is needed to prepare 50.0 mL of 1.00 M solution?
A) 6.42 g
B) 12.7 g
C) 14.3 g
D) 9.86 g
E) none of these
A) 6.42 g
B) 12.7 g
C) 14.3 g
D) 9.86 g
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
39
What is the molarity of a solution prepared by dissolving 47 g of KCl in enough water to give 375 mL of solution?
Molar Mass(KCl) = 74.55 g/mol
A) 0.236 M
B) 0.595 M
C) 1.68 M
D) 4.23 M
E) 9.34 M
Molar Mass(KCl) = 74.55 g/mol
A) 0.236 M
B) 0.595 M
C) 1.68 M
D) 4.23 M
E) 9.34 M

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
40
What are the spectator ions and what is the net ionic equation for the following double-replacement, precipitation reaction?
Na2S (aq) + ZnSO4 (aq)→Spectator Ions Net reaction
I. Zn2+ and S2 - 2 Na+ (aq) + SO42 - (aq)→Na2SO4 (aq)
II. Na+ and SO42 - Zn2+ (aq) + S2 - (aq)→ZnS (s)
III. Na+ and Zn2+ S2 - (aq) + SO42 - (aq)→ S2O44 - (aq)
IV. S2 - and SO42 - Na+ (aq) + Zn2+ (aq)→NaZn3+ (aq)
V. Na+ and S2 - Zn2+ (aq) + SO42 - (aq)→ZnSO4 (s)
A) I
B) II
C) III
D) IV
E) V
Na2S (aq) + ZnSO4 (aq)→Spectator Ions Net reaction
I. Zn2+ and S2 - 2 Na+ (aq) + SO42 - (aq)→Na2SO4 (aq)
II. Na+ and SO42 - Zn2+ (aq) + S2 - (aq)→ZnS (s)
III. Na+ and Zn2+ S2 - (aq) + SO42 - (aq)→ S2O44 - (aq)
IV. S2 - and SO42 - Na+ (aq) + Zn2+ (aq)→NaZn3+ (aq)
V. Na+ and S2 - Zn2+ (aq) + SO42 - (aq)→ZnSO4 (s)
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
41
How many grams of NaCl are required to prepare 500. mL of an aqueous 0.500 M NaCl solution?
MM(NaCl) = 58.44 g/mol
A) 4.28 g NaCl
B) 14.6 g NaCl
C) 58.4 g NaCl
D) 62.8 g NaCl
E) 232 g NaCl
MM(NaCl) = 58.44 g/mol
A) 4.28 g NaCl
B) 14.6 g NaCl
C) 58.4 g NaCl
D) 62.8 g NaCl
E) 232 g NaCl

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
42
How many moles of H2SO4 are present in 211 mL of a 4.36 molar solution?
A) 1.12 mol
B) 0.342 mol
C) 0.920 mol
D) 1.42 mol
E) none of these
A) 1.12 mol
B) 0.342 mol
C) 0.920 mol
D) 1.42 mol
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
43
How many grams of NH3 are present in 2.50 L of 3.00 M NH3 solution?
Molar Mass(NH3) = 17.03 g/mol
A) 0.0705 g NH3
B) 7.50 g NH3
C) 14.2 g NH3
D) 20.4 g NH3
E) 128 g NH3
Molar Mass(NH3) = 17.03 g/mol
A) 0.0705 g NH3
B) 7.50 g NH3
C) 14.2 g NH3
D) 20.4 g NH3
E) 128 g NH3

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
44
How many grams of HCl are present in 2.50 L of 3.00 M HCl solution?
Molar Mass(HCl) = 36.46 g/mol
A) 0.0228 g HCl
B) 0.206 g HCl
C) 30.4 g HCl
D) 273 g HCl
E) None of these
Molar Mass(HCl) = 36.46 g/mol
A) 0.0228 g HCl
B) 0.206 g HCl
C) 30.4 g HCl
D) 273 g HCl
E) None of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
45
How many milliliters of 1.50 M KOH solution are needed to provide 0.125 moles of KOH?
A) 0.0833 mL
B) 12.0 mL
C) 83.3 mL
D) 188 mL
E) 1.20×104 mL
A) 0.0833 mL
B) 12.0 mL
C) 83.3 mL
D) 188 mL
E) 1.20×104 mL

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
46
What volume in milliliters of a 0.200 M NaCl solution is required to provide 1.00 grams of NaCl?
Molar Mass(NaCl) = 58.44 g/mol
A) 3.42 mL of 0.200 M NaCl
B) 11.7 mL of 0.200 M NaCl
C) 85.6 mL of 0.200 M NaCl
D) 292 mL of 0.200 M NaCl
E) 5000 mL of 0.200 M NaCl
Molar Mass(NaCl) = 58.44 g/mol
A) 3.42 mL of 0.200 M NaCl
B) 11.7 mL of 0.200 M NaCl
C) 85.6 mL of 0.200 M NaCl
D) 292 mL of 0.200 M NaCl
E) 5000 mL of 0.200 M NaCl

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
47
How many moles of KNO3 are present in 45.6 mL of a 3.11 molar solution?
A) 0.142 mol
B) 14.3 mol
C) 3.11 mol
D) 142 mol
E) none of these
A) 0.142 mol
B) 14.3 mol
C) 3.11 mol
D) 142 mol
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
48
How many grams of NaF are needed to make 250 mL of a 0.22 molar solution?
A) 7.2 g
B) 1.2 g
C) 2.3 g
D) 4.6 g
E) none of these
A) 7.2 g
B) 1.2 g
C) 2.3 g
D) 4.6 g
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
49
How many grams of KCl is required to prepared 500 mL of a 2.50 M aqueous solution of KCl?
Molar Mass(KCl) = 74.55 g/mol
A) 0.0168 g KCl
B) 0.0671 g KCl
C) 14.9 g KCl
D) 93.2 g KCl
E) 373 g KCl
Molar Mass(KCl) = 74.55 g/mol
A) 0.0168 g KCl
B) 0.0671 g KCl
C) 14.9 g KCl
D) 93.2 g KCl
E) 373 g KCl

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
50
How many grams of H2SO4 are present in 75.0 mL of 12.0 M H2SO4?
Molar Mass (H2SO4) = 98.09 g/mol
A) 1.63 g H2SO4
B) 9.18 g H2SO4
C) 15.7 g H2SO4
D) 88.3 g H2SO4
E) 613 g H2SO4
Molar Mass (H2SO4) = 98.09 g/mol
A) 1.63 g H2SO4
B) 9.18 g H2SO4
C) 15.7 g H2SO4
D) 88.3 g H2SO4
E) 613 g H2SO4

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
51
How many moles of OH - ions are present in 422 mL of a 1.21 molar solution of Ba(OH)2?
A) 2.22 mol
B) 0.312 mol
C) 0.511 mol
D) 1.02 mol
E) none of these
A) 2.22 mol
B) 0.312 mol
C) 0.511 mol
D) 1.02 mol
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
52
How many moles of NO3 - ions are present in 533 mL of a 0.111 molar solution of Ca(NO3)2?
A) 0.0300 mol
B) 0.118 mol
C) 0.0592 mol
D) 1.22 mol
E) none of these
A) 0.0300 mol
B) 0.118 mol
C) 0.0592 mol
D) 1.22 mol
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
53
How many grams of AgNO3 are present in 50.0 mL of a 12.0 M AgNO3 solution?
Molar Mass(AgNO3) = 169.88 g/mol
A) 3.53 g of AgNO3
B) 40.8 g of AgNO3
C) 102 g of AgNO3
D) 283 g of AgNO3
E) 708 g of AgNO3
Molar Mass(AgNO3) = 169.88 g/mol
A) 3.53 g of AgNO3
B) 40.8 g of AgNO3
C) 102 g of AgNO3
D) 283 g of AgNO3
E) 708 g of AgNO3

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
54
How many grams of sodium chloride are needed to prepare 1.4 L of a 1.0 molar solution of sodium chloride?
A) 82 g
B) 44 g
C) 37 g
D) 2.4 g
E) none of these
A) 82 g
B) 44 g
C) 37 g
D) 2.4 g
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
55
How many moles of Na3PO4 are present in 5.00 mL of a 0.226 molar solution?
A) 1.13×10 - 3 mol
B) 1.67×10 - 3 mol
C) 3.33×10 - 3 mol
D) 0.00224 mol
E) none of these
A) 1.13×10 - 3 mol
B) 1.67×10 - 3 mol
C) 3.33×10 - 3 mol
D) 0.00224 mol
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
56
How many moles of Ca2+ ions are present in 15.6 mL of a 1.21 molar solution of CaCl2?
A) 0.0189 mol
B) 0.0112 mol
C) 0.102 mol
D) 0.00332 mol
E) none of these
A) 0.0189 mol
B) 0.0112 mol
C) 0.102 mol
D) 0.00332 mol
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
57
How many grams of potassium sulfate are needed to prepare 2.44 L of a 0.0333 molar solution of potassium sulfate?
A) 8.72 g
B) 4.04 g
C) 7.22 g
D) 14.2 g
E) none of these
A) 8.72 g
B) 4.04 g
C) 7.22 g
D) 14.2 g
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
58
How many grams of solute is present in 164.1 mL of 0.207 M calcium acetate, Ca(C2H3O2)2?
Molar Mass(Ca(C2H3O2)2) = 158.17 g/mol
A) 0.200 g Ca(C2H3O2)2
B) 0.215 g Ca(C2H3O2)2
C) 4.66 g Ca(C2H3O2)2
D) 5.01 g Ca(C2H3O2)2
E) 5.37 g Ca(C2H3O2)2
Molar Mass(Ca(C2H3O2)2) = 158.17 g/mol
A) 0.200 g Ca(C2H3O2)2
B) 0.215 g Ca(C2H3O2)2
C) 4.66 g Ca(C2H3O2)2
D) 5.01 g Ca(C2H3O2)2
E) 5.37 g Ca(C2H3O2)2

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
59
How many moles of K+ ions are present in 2.00 L of a 0.211 molar solution of K2SO4?
A) 0.422 mol
B) 0.844 mol
C) 0.211 mol
D) 0.522 mol
E) none of these
A) 0.422 mol
B) 0.844 mol
C) 0.211 mol
D) 0.522 mol
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
60
How many grams of silver nitrate (AgNO3) are present in 255 mL of 2.50 M silver nitrate solution?
Molar Mass(AgNO3) = 169.87 g/mol
A) 5.77×10 - 2 g AgNO3
B) 17.3 g AgNO3
C) 108 g AgNO3
D) 266 g AgNO3
E) 1.67×103 g AgNO3
Molar Mass(AgNO3) = 169.87 g/mol
A) 5.77×10 - 2 g AgNO3
B) 17.3 g AgNO3
C) 108 g AgNO3
D) 266 g AgNO3
E) 1.67×103 g AgNO3

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
61
What mass of BaSO4 precipitates from mixing 2.44 L of 0.212 molar BaCl2 with excess H2SO4?
A) 121 g
B) 50.3 g
C) 241 g
D) 101 g
E) none of these
A) 121 g
B) 50.3 g
C) 241 g
D) 101 g
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
62
What is the final concentration of a NaCl aqueous solution prepared by mixing 20.0 mL of water witH₆0.0 mL of 2.00 M NaCl solution?
A) 0.667 M
B) 1.50 M
C) 2.67 M
D) 3.00 M
E) 6.00 M
A) 0.667 M
B) 1.50 M
C) 2.67 M
D) 3.00 M
E) 6.00 M

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
63
Concentrated sulfuric acid, H2SO4, is 18.0 M. How many milliliters of concentrated sulfuric acid are required to prepare 500 mL of 3.60 M H2SO4?
A) 0.400 mL
B) 10.0 mL
C) 100 mL
D) 400 mL
E) 2500 mL
A) 0.400 mL
B) 10.0 mL
C) 100 mL
D) 400 mL
E) 2500 mL

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
64
How many mL of 10. molar HCl is needed to make up a 200 mL solution oF4.0 molar HCl?
A) 160 mL
B) 40 mL
C) 80 mL
D) 20 mL
E) none of these
A) 160 mL
B) 40 mL
C) 80 mL
D) 20 mL
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
65
What volume of a 2.00 molar standard solution of NaOH is needed to prepare 1.00 L of a 0.0250 molar solution?
A) 12.5 mL
B) 25.0 mL
C) 6.25 mL
D) 18.2 mL
E) none of these
A) 12.5 mL
B) 25.0 mL
C) 6.25 mL
D) 18.2 mL
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
66
What is the final concentration of a NaCl solution prepared by adding 20.0 mL of water to 30.0 mL of 5.0 M NaCl solution?
A) 2.00 M NaCl
B) 3.00 M NaCl
C) 3.33 M NaCl
D) 5.00 M NaCl
E) 7.50 M NaCl
A) 2.00 M NaCl
B) 3.00 M NaCl
C) 3.33 M NaCl
D) 5.00 M NaCl
E) 7.50 M NaCl

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
67
What volume of 1.1 M HCl is needed to prepare 100 mL of a 0.20 M HCl solution?
A) 9.0 mL
B) 18 mL
C) 32 mL
D) 36 mL
E) none of these
A) 9.0 mL
B) 18 mL
C) 32 mL
D) 36 mL
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
68
How many milliliters of water must be added to 75.0 mL of 0.150 M AgNO3 in order to prepare a 0.100 M solution?
(Assume volumes are additive.)
A) 25.0 mL of H2O
B) 38.0 mL of H2O
C) 50.0 mL of H2O
D) 113 mL of H2O
E) 188 mL of H2O
(Assume volumes are additive.)
A) 25.0 mL of H2O
B) 38.0 mL of H2O
C) 50.0 mL of H2O
D) 113 mL of H2O
E) 188 mL of H2O

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
69
What mass of AgCl can be formed from the reaction of excess AgNO3 witH₂ 00 mL of 0.500 M CaCl2?
A) 28.7 g
B) 14.4 g
C) 57.4 g
D) 20.2 g
E) none of these
A) 28.7 g
B) 14.4 g
C) 57.4 g
D) 20.2 g
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
70
How many milliliters of water should be added to 25.0 mL of 1.00 M AgNO3 in order to provide a 0.125 M AgNO3 solution?
A) 3.13 mL of water
B) 28.1 mL of water
C) 175 mL of water
D) 200 mL of water
E) 225 mL of water
A) 3.13 mL of water
B) 28.1 mL of water
C) 175 mL of water
D) 200 mL of water
E) 225 mL of water

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
71
How many milliliters of concentrated, 18.0 M H2SO4 are required to prepare 250 mL of 3.00 M H2SO4?
A) 0.667 mL
B) 24.0 mL
C) 41.7 mL
D) 1500 mL
E) None of these
A) 0.667 mL
B) 24.0 mL
C) 41.7 mL
D) 1500 mL
E) None of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
72
What is the molarity of a solution of H2SO4 that is prepared by the dilution of 150 mL of 5.00 molar HCl to 2.00L?
A) 0.500 molar
B) 0.375 molar
C) 0.750 molar
D) 0.150 molar
E) none of these
A) 0.500 molar
B) 0.375 molar
C) 0.750 molar
D) 0.150 molar
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
73
What mass of AgCl is produced by mixing 2.4 g of CaCl2 with 30 mL of a 2.4 M AgNO3 solution?
A) 10 g
B) 6.2 g
C) 3.1 g
D) 5.0 g
E) none of these
A) 10 g
B) 6.2 g
C) 3.1 g
D) 5.0 g
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
74
What is the molarity of a solution prepared by diluting 50.0 mL of 0.110 M HCl to a final volume of 75 mL?
A) 0.044 M
B) 0.066 M
C) 0.0733 M
D) 0.165 M
E) None of these
A) 0.044 M
B) 0.066 M
C) 0.0733 M
D) 0.165 M
E) None of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
75
What is the molarity of a solution prepared by diluting 25.0 mL of 0.220 M NaCl to a final volume of 75.0 mL?
A) 0.0733 M NaCl
B) 0.220 M NaCl
C) 0.660 M NaCl
D) 1.52 M NaCl
E) 13.6 M NaCl
A) 0.0733 M NaCl
B) 0.220 M NaCl
C) 0.660 M NaCl
D) 1.52 M NaCl
E) 13.6 M NaCl

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
76
What mass of AgCl forms from the reaction of excess AgNO3 witH50 mL of 0.20 M solution of BaCl2?
A) 1.4 g
B) 2.9 g
C) 0.70 g
D) 3.4 g
E) none of these
A) 1.4 g
B) 2.9 g
C) 0.70 g
D) 3.4 g
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
77
What mass of Al(OH)3 is formed from mixing 1.24 L of 0.332 molar AlCl3 with excess NaOH?
A) 12.1 g
B) 40.3 g
C) 24.1 g
D) 32.1 g
E) none of these
A) 12.1 g
B) 40.3 g
C) 24.1 g
D) 32.1 g
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
78
What is the molarity of a solution prepared by diluting 25 mL of 1.2 molar H2SO4 to 1.0 L?
A) 0.015 molar
B) 0.060 molar
C) 0.030 molar
D) 0.040 molar
E) none of these
A) 0.015 molar
B) 0.060 molar
C) 0.030 molar
D) 0.040 molar
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
79
What is the molarity of a solution of HCl that is prepared by the dilution of 10 mL of 2.00 molar HCl to 1.00L?
A) 0.500 molar
B) 0.0350 molar
C) 0.0200 molar
D) 0.100 molar
E) none of these
A) 0.500 molar
B) 0.0350 molar
C) 0.0200 molar
D) 0.100 molar
E) none of these

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
80
What is the molarity of a solution prepared by diluting 75 mL of 2.35 M Na2SO4 solution to a final volume of 250 mL?
A) 0.0441 M
B) 0.128 M
C) 0.705 M
D) 1.42 M
E) 7.83 M
A) 0.0441 M
B) 0.128 M
C) 0.705 M
D) 1.42 M
E) 7.83 M

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck


