Deck 1: Introduction to Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/95
Play
Full screen (f)
Deck 1: Introduction to Chemistry
1
A glass of tea with a uniform composition throughout would be an example of a(n) ____.
A) pure substance
B) element
C) compound
D) homogeneous mixture
E) heterogeneous mixture
A) pure substance
B) element
C) compound
D) homogeneous mixture
E) heterogeneous mixture
homogeneous mixture
2
Which of the following is a heterogeneous mixture?
A) salt water
B) salt
C) milk
D) sand
E) water
A) salt water
B) salt
C) milk
D) sand
E) water
sand
3
Which of the following is not a chemical change?
A) developing a film
B) refrigerating milk
C) eating beef
D) extinguishing a fire
E) frying an egg
A) developing a film
B) refrigerating milk
C) eating beef
D) extinguishing a fire
E) frying an egg
refrigerating milk
4
Which of the following is correctly classified as a hypothesis?
A) "The sun will rise in the East today."
B) "Global warming is a result of the increased carbon dioxide concentration in the atmosphere."
C) "Our team is better than theirs."
D) "Cookies will turn brown when baked."
E) "My book is better than yours."
A) "The sun will rise in the East today."
B) "Global warming is a result of the increased carbon dioxide concentration in the atmosphere."
C) "Our team is better than theirs."
D) "Cookies will turn brown when baked."
E) "My book is better than yours."

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
5
Which form of substances listed below cannot be separated into simpler substances by chemical or physical means?
A) an element
B) a compound
C) a heterogeneous mixture
D) a homogeneous mixture
E) All substances can be separated into simpler substances.
A) an element
B) a compound
C) a heterogeneous mixture
D) a homogeneous mixture
E) All substances can be separated into simpler substances.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following represents a chemical change ?
A) Cutting a string into two pieces.
B) Melting candle wax.
C) Evaporation of water from a lake.
D) Rusting of an iron nail.
E) Peeling an apple.
A) Cutting a string into two pieces.
B) Melting candle wax.
C) Evaporation of water from a lake.
D) Rusting of an iron nail.
E) Peeling an apple.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
7
All of the following are properties of Magnesium. Which would be considered a chemical property of magnesium?
I. Magnesium burns in air to produce a white substance known as magnesium oxide.
II. Magnesium dissolves in acidic water to produce hydrogen gas.
III. The density of magnesium is 1.738 g/mL.
A) I
B) II
C) III
D) I and II
E) all of these
I. Magnesium burns in air to produce a white substance known as magnesium oxide.
II. Magnesium dissolves in acidic water to produce hydrogen gas.
III. The density of magnesium is 1.738 g/mL.
A) I
B) II
C) III
D) I and II
E) all of these

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
8
Which does not represent a scientific investigation?
A) Weighing the results of an experiment to obtain quantitative data.
B) Weighing lab reports on a chemical balance to determine the grade.
C) Graphing the mass of a product as a function of the mass of the starting material.
D) Determining the temperature at which the maximal amount of product is obtained.
E) Trying different conditions if an initial experiment fails.
A) Weighing the results of an experiment to obtain quantitative data.
B) Weighing lab reports on a chemical balance to determine the grade.
C) Graphing the mass of a product as a function of the mass of the starting material.
D) Determining the temperature at which the maximal amount of product is obtained.
E) Trying different conditions if an initial experiment fails.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is not a physical change?
A) ice melting
B) dry ice subliming
C) a light-bulb glowing
D) beef cooking
E) distilling alcohol
A) ice melting
B) dry ice subliming
C) a light-bulb glowing
D) beef cooking
E) distilling alcohol

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following would be considered a chemical change ?
I. Frost forms on the window
II. A Silver fork tarnishes
III. Toasting a slice of bread.
A) I only
B) I and II
C) I and III
D) II and III
E) All of these
I. Frost forms on the window
II. A Silver fork tarnishes
III. Toasting a slice of bread.
A) I only
B) I and II
C) I and III
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
11
All of the following are properties of carbon dioxide, CO2. Which of the following is a chemical property of this substance?
A) It reacts with water to form carbonic acid.
B) It is a colorless gas at room temperature and 1 atm pressure.
C) It is a solid below - 78 ° C.
D) Its density as a solid equals 1.35 g/mL.
E) None of these is a chemical property.
A) It reacts with water to form carbonic acid.
B) It is a colorless gas at room temperature and 1 atm pressure.
C) It is a solid below - 78 ° C.
D) Its density as a solid equals 1.35 g/mL.
E) None of these is a chemical property.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following studies is not considered to be "Chemistry?"
A) Studying the interactions between energy and matter.
B) Classifying elements into metallic, nonmetallic or semimetallic.
C) Studying the interactions between matter and other matter
D) Removing harmful impurities from a synthetic drug.
E) Separating the light spectrum into its component colors.
A) Studying the interactions between energy and matter.
B) Classifying elements into metallic, nonmetallic or semimetallic.
C) Studying the interactions between matter and other matter
D) Removing harmful impurities from a synthetic drug.
E) Separating the light spectrum into its component colors.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
13
Of the three properties listed below, which ones are intensive properties?
I. mass
II. density
III. color
A) I and II
B) II and III
C) II only
D) I only
E) I, II, and III
I. mass
II. density
III. color
A) I and II
B) II and III
C) II only
D) I only
E) I, II, and III

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is considered a chemical change ?
A) Beef is browned by heating in a skillet.
B) A blackberry changes colors from red to black.
C) Addition of hydrogen peroxide to an open cut produces a white liquid and fizzing.
D) a and b
E) all of these.
A) Beef is browned by heating in a skillet.
B) A blackberry changes colors from red to black.
C) Addition of hydrogen peroxide to an open cut produces a white liquid and fizzing.
D) a and b
E) all of these.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
15
"A 33 g sample of red liquid at its freezing point of 260 K had a density of 0.926 g/mL." Which of the properties given in the previous statement is extensive ?
A) 33 g
B) red
C) freezing point
D) 260 K
E) 0.926 g/mL
A) 33 g
B) red
C) freezing point
D) 260 K
E) 0.926 g/mL

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following would be considered a physical change ?
A) gasoline burning
B) salt dissolving in water
C) food spoiling
D) iron rusting
E) a battery discharging
A) gasoline burning
B) salt dissolving in water
C) food spoiling
D) iron rusting
E) a battery discharging

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
17
The following are properties of sodium metal. Which would be considered a chemical property of sodium metal?
A) Sodium is a silvery-white metal
B) Sodium has a melting point of 98 ° C
C) Sodium has a density equal to 0.968 g/mL.
D) Sodium reacts with water violently to produce hydrogen gas.
E) Sodium metal is drawn into a magnetic field.
A) Sodium is a silvery-white metal
B) Sodium has a melting point of 98 ° C
C) Sodium has a density equal to 0.968 g/mL.
D) Sodium reacts with water violently to produce hydrogen gas.
E) Sodium metal is drawn into a magnetic field.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
18
How can "oil and vinegar" be classified?
(Two phases are present.)
A) pure substance
B) element
C) compound
D) homogeneous mixture
E) heterogeneous mixture
(Two phases are present.)
A) pure substance
B) element
C) compound
D) homogeneous mixture
E) heterogeneous mixture

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following processes is considered a chemical change ?
I. Corrosion of aluminum metal
II. Digesting a candy bar.
III. Melting of ice.
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. Corrosion of aluminum metal
II. Digesting a candy bar.
III. Melting of ice.
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
20
Identify the matter that is correctly classified as a mixture.
A) ethyl alcohol
B) carbon dioxide
C) water
D) tin
E) air
A) ethyl alcohol
B) carbon dioxide
C) water
D) tin
E) air

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
21
A compound is:
A) a pure substance that can be decomposed chemically.
B) a characteristic that describes a substance.
C) a pure substance that cannot be decomposed chemically.
D) a combination of atoms.
E) none of these.
A) a pure substance that can be decomposed chemically.
B) a characteristic that describes a substance.
C) a pure substance that cannot be decomposed chemically.
D) a combination of atoms.
E) none of these.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is an example of a homogeneous mixture ?
I. Water and dissolved salt
II. Water and sand
III. Carbonated water (soda) and ice
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. Water and dissolved salt
II. Water and sand
III. Carbonated water (soda) and ice
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following best describes an element?
A) An element is a mixture that can be separated into simpler substances using physical methods .
B) An element is a mixture that can be separated into simpler substances using chemical methods .
C) An element is a pure substance that can be separated into simpler substances using physical methods .
D) An element is a pure substance that can be separated into simpler substances using chemical methods .
E) An element is a pure substance that cannot be separated into simpler substances using ordinary physical or chemical methods.
A) An element is a mixture that can be separated into simpler substances using physical methods .
B) An element is a mixture that can be separated into simpler substances using chemical methods .
C) An element is a pure substance that can be separated into simpler substances using physical methods .
D) An element is a pure substance that can be separated into simpler substances using chemical methods .
E) An element is a pure substance that cannot be separated into simpler substances using ordinary physical or chemical methods.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following volume measurements is the least precise measurement?
A) 1.0 mL
B) 2.018 mL
C) 2.02 mL
D) 2.022 mL
E) 3 mL
A) 1.0 mL
B) 2.018 mL
C) 2.02 mL
D) 2.022 mL
E) 3 mL

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following matched pairs of elemental names and chemical symbols is properly labeled?
I. Calcium, C
II. Plutonium, Pt
III. Silicon, Si
IV. Iron, I
V. Nitrogen, N
A) I, IV and V
B) II, III and V
C) I, III, IV and V
D) III and V
E) All are properly labeled
I. Calcium, C
II. Plutonium, Pt
III. Silicon, Si
IV. Iron, I
V. Nitrogen, N
A) I, IV and V
B) II, III and V
C) I, III, IV and V
D) III and V
E) All are properly labeled

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
26
Three students measured the density of pure water in the laboratory. Their results are as follows:
Student 1:
Density = 1.20 g/mL Student 2:
Density = 1.21 g/mL Student 3:
Density = 1.19 g/mL The actual density of water at 25 ° C equals 0.997 g/mL. What do the results of these three experiments demonstrate when compared to the true density of water?
A) High accuracy and high precision
B) High accuracy and low precision
C) Low accuracy and high precision
D) Low accuracy and low precision
E) None of these
Student 1:
Density = 1.20 g/mL Student 2:
Density = 1.21 g/mL Student 3:
Density = 1.19 g/mL The actual density of water at 25 ° C equals 0.997 g/mL. What do the results of these three experiments demonstrate when compared to the true density of water?
A) High accuracy and high precision
B) High accuracy and low precision
C) Low accuracy and high precision
D) Low accuracy and low precision
E) None of these

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
27
A precise measurement
A) is known to two or more significant figures.
B) is known to two or more decimal places.
C) is repeatable.
D) is close to the correct answer
E) is beyond the scope of a first-year chemistry student.
A) is known to two or more significant figures.
B) is known to two or more decimal places.
C) is repeatable.
D) is close to the correct answer
E) is beyond the scope of a first-year chemistry student.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
28
What is the chemical symbol for the element tin ?
A) T
B) Sn
C) Ti
D) Tn
E) St
A) T
B) Sn
C) Ti
D) Tn
E) St

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
29
In the reported measurement of 24.0 mm, what digit is uncertain ?
A) tens place
B) in the ones place
C) in the tenths place
D) in the hundredths place
E) All places are reported with certainty.
A) tens place
B) in the ones place
C) in the tenths place
D) in the hundredths place
E) All places are reported with certainty.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
30
Three students measured the freezing temperature of water in the laboratory. Their results are as follows:
Student 1:
Freezing temperature = 2.86 ° C Student 2:
Freezing temperature = - 4.52 ° C Student 3:
Freezing temperature = 3.21 ° C The actual freezing temperature of water equals 0.00 ° C. The results of these three students when compared to the true freezing temperature of water demonstrates:
A) High accuracy and high precision
B) High accuracy and low precision
C) Low accuracy and high precision
D) low accuracy and low precision
E) None of these
Student 1:
Freezing temperature = 2.86 ° C Student 2:
Freezing temperature = - 4.52 ° C Student 3:
Freezing temperature = 3.21 ° C The actual freezing temperature of water equals 0.00 ° C. The results of these three students when compared to the true freezing temperature of water demonstrates:
A) High accuracy and high precision
B) High accuracy and low precision
C) Low accuracy and high precision
D) low accuracy and low precision
E) None of these

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following best describes a mixture ?
A) It's composition can vary but its properties remain constant .
B) It's composition and properties can vary .
C) It's composition remains constant but its properties varies .
D) It's composition and properties remains constant .
A) It's composition can vary but its properties remain constant .
B) It's composition and properties can vary .
C) It's composition remains constant but its properties varies .
D) It's composition and properties remains constant .

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
32
What length in millimeters can be reported for the rectangle below?
(Note that the ruler measures in centimeters.)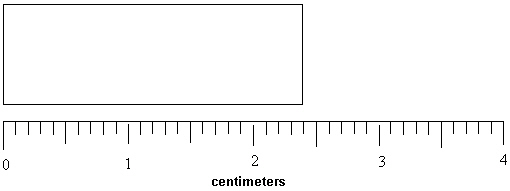
A) 24 mm
B) 24.0 mm
C) 2.4 mm
D) 2.40 mm
E) 240 mm
(Note that the ruler measures in centimeters.)

A) 24 mm
B) 24.0 mm
C) 2.4 mm
D) 2.40 mm
E) 240 mm

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
33
The definition "stable pure substance that cannot be separated into simpler substances by chemical means" would best fit which term listed below?
A) element
B) compound
C) atom
D) molecule
E) xanon
A) element
B) compound
C) atom
D) molecule
E) xanon

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following measurements is considered a combination of one of the seven base units from which all other measurements can be made?
A) mass
B) volume
C) amount
D) temperature
E) time
A) mass
B) volume
C) amount
D) temperature
E) time

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following matched pairs of elemental names and chemical symbols is properly labeled?
I. Magnesium, Mg
II. Mercury, M
III. Silver, Si
IV. Sodium, S
V. Oxygen, O
A) I only
B) II and III
C) I and V
D) I, IV and V
E) All are properly labeled
I. Magnesium, Mg
II. Mercury, M
III. Silver, Si
IV. Sodium, S
V. Oxygen, O
A) I only
B) II and III
C) I and V
D) I, IV and V
E) All are properly labeled

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
36
The elements Carbon, Lead, and Magnesium are designated by what chemical symbols respectively?
A) C, L, M
B) Ca, Le, Ma
C) C, Pb, Mn
D) C, Pb, Mg
E) Ca, Pb, Mn
A) C, L, M
B) Ca, Le, Ma
C) C, Pb, Mn
D) C, Pb, Mg
E) Ca, Pb, Mn

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
37
Which is properly classified as a compound?
A) salt water
B) salt
C) wine
D) bread
E) copper
A) salt water
B) salt
C) wine
D) bread
E) copper

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
38
An accurate measurement
A) is known to two or more significant figures.
B) is known to two or more decimal places.
C) is repeatable.
D) is close to the correct answer
E) is beyond the scope of a first year chemistry student.
A) is known to two or more significant figures.
B) is known to two or more decimal places.
C) is repeatable.
D) is close to the correct answer
E) is beyond the scope of a first year chemistry student.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is not an element?
A) niobium
B) thallium
C) silicon
D) sodium
E) ammonium
A) niobium
B) thallium
C) silicon
D) sodium
E) ammonium

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following best describes a compound ?
A) A compound is a mixture that can be separated into its constituent elements using physical methods .
B) A compound is a mixture that can be separated into its constituent elements using chemical methods .
C) A compound is a pure substance that can be separated into its constituent elements using physical methods .
D) A compound is a pure substance that can be separated into its constituent elements using chemical methods .
A) A compound is a mixture that can be separated into its constituent elements using physical methods .
B) A compound is a mixture that can be separated into its constituent elements using chemical methods .
C) A compound is a pure substance that can be separated into its constituent elements using physical methods .
D) A compound is a pure substance that can be separated into its constituent elements using chemical methods .

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
41
What prefix is used to indicate a base unit multiplied by 10 - 6?
A) kilo
B) mega
C) micro
D) centi
E) deca
A) kilo
B) mega
C) micro
D) centi
E) deca

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
42
Two students combined two different volumes of water. The first student measured 25.4 mL of water using a 100.0 mL graduated cylinder and the second student measured 0.15 mL of water using a 50.00 mL burette. What total volume should be reported after taking into account significant digits and the convention used for rounding?
A) 25 mL
B) 25.5 mL
C) 25.55 mL
D) 25.6 mL
E) 26 mL
A) 25 mL
B) 25.5 mL
C) 25.55 mL
D) 25.6 mL
E) 26 mL

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
43
How would the measurement, 5125 m, be rounded off to three significant digits and expressed in scientific notation ?
A) 5.12×10 - 3 m
B) 5.13×10 - 3 m
C) 5.130×10 - 3 m
D) 5.12×103 m
E) 5.13×104 m
A) 5.12×10 - 3 m
B) 5.13×10 - 3 m
C) 5.130×10 - 3 m
D) 5.12×103 m
E) 5.13×104 m

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
44
Which metric prefix listed below has a power of 10 - 12 associated with it?
A) centi
B) micro
C) milli
D) kilo
E) pico
A) centi
B) micro
C) milli
D) kilo
E) pico

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
45
Three problems are worked below. Choose the answer that properly describes the correct use of significant digits
I. 0.34
II. 14.3
III. 14.21 +14.2×2.0×134 14.5 28.6 1.90×103
A) I and II are correct, III is incorrect
B) I and III are correct, II is incorrect
C) II and III are correct, I is incorrect
D) all three are correct
E) none of these
I. 0.34
II. 14.3
III. 14.21 +14.2×2.0×134 14.5 28.6 1.90×103
A) I and II are correct, III is incorrect
B) I and III are correct, II is incorrect
C) II and III are correct, I is incorrect
D) all three are correct
E) none of these

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
46
The number 0.00042 is equal to:
A) 4.2×104
B) 4.2×10 - 4
C) 4.2×103
D) 4.2×10 - 3
E) 4.2×10 - 2
A) 4.2×104
B) 4.2×10 - 4
C) 4.2×103
D) 4.2×10 - 3
E) 4.2×10 - 2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
47
What digit(s) is(are) considered the digit of uncertainty in the following measurement?
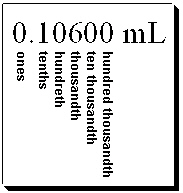
A) thousandths place
B) ones, hundredths, ten thousandths and hundred thousandths places
C) ten thousandths place
D) hundred thousandths place
E) millionth place

A) thousandths place
B) ones, hundredths, ten thousandths and hundred thousandths places
C) ten thousandths place
D) hundred thousandths place
E) millionth place

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
48
Which is an abbreviation for an SI base unit?
A) J
B) kg
C) Nt
D) ° C
E) L
A) J
B) kg
C) Nt
D) ° C
E) L

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
49
Which measurements below have four significant digits?
Measurement
I. 0.023 mL
II. 2300 mL
III. 1700 . mL
IV. 0.004050 mL
A) all of these have four significant digits
B) I, II and III
C) II, III, and IV
D) I and II
E) III and IV
Measurement
I. 0.023 mL
II. 2300 mL
III. 1700 . mL
IV. 0.004050 mL
A) all of these have four significant digits
B) I, II and III
C) II, III, and IV
D) I and II
E) III and IV

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
50
The dimensions of the following cube were measured. What would be the volume of this cube expressed to the proper number of significant digits?
(Volume = l w d )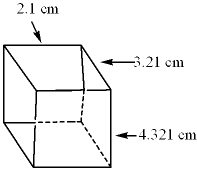
A) 29.127861 cm3
B) 29.13 cm3
C) 29.1 cm3
D) 29. cm3
E) 30 cm3
(Volume = l w d )

A) 29.127861 cm3
B) 29.13 cm3
C) 29.1 cm3
D) 29. cm3
E) 30 cm3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
51
What prefix is used to indicate a base unit multiplied by 10 - 1?
A) deca
B) centi
C) milli
D) deci
E) micro
A) deca
B) centi
C) milli
D) deci
E) micro

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
52
Choose the best answer to the following math problem ( with the proper number of significant digits and rounded correctly ). (12.3432 - 8.84)×22.48 = ??
A) 78.751936
B) 78.75
C) 78.76
D) 78.8
E) 78.752
A) 78.751936
B) 78.75
C) 78.76
D) 78.8
E) 78.752

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
53
What is the density of mercury when reported to the proper number of significant digits and in scientific notation if a sample is found to have a mass of 524.5 g and occupies a volume of 38.72 ml?
A) 7.38×10 - 2 g/mL
B) 7.382×10 - 2 g/mL
C) 1.35×101 g/mL
D) 1.354×101 g/mL
E) 1.355×101 g/mL
A) 7.38×10 - 2 g/mL
B) 7.382×10 - 2 g/mL
C) 1.35×101 g/mL
D) 1.354×101 g/mL
E) 1.355×101 g/mL

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
54
How many significant digits are there in 0.0050 mL?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
55
The correct answer for (1.22×103)×0.013 is:
A) 15.8
B) 158
C) 16
D) 15.86
E) 93846
A) 15.8
B) 158
C) 16
D) 15.86
E) 93846

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
56
Which metric prefix(es) listed below is(are) correctly assigned the proper power of ten?
I. giga:
109
II. micro:
106
III. nano:
10 - 12
A) I only
B) II only
C) III only
D) I and II
E) II and III
I. giga:
109
II. micro:
106
III. nano:
10 - 12
A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
57
The number 486,000 is equal to:
A) 4.86×102
B) 4.86×10 - 4
C) 4.86×103
D) 4.86×104
E) 4.86×105
A) 4.86×102
B) 4.86×10 - 4
C) 4.86×103
D) 4.86×104
E) 4.86×105

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
58
What is the temperature given by the thermometer below written with the proper number of significant digits?

A) 32.3 ° C
B) 32.30 ° C
C) 32 ° C
D) 32.300 ° C
E) 30 ° C

A) 32.3 ° C
B) 32.30 ° C
C) 32 ° C
D) 32.300 ° C
E) 30 ° C

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
59
Which metric prefix(es) listed below is(are) correctly assigned the proper power of ten?
I. deci:
101
II. nano:
10 - 6
III. mega:
106
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. deci:
101
II. nano:
10 - 6
III. mega:
106
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
60
Properly expressed, the answer to the mathematical manipulation (0.243×4.2) + 21 = is:
A) 22
B) 22.0
C) 2.2×102
D) 22.02
E) 2.202×102
A) 22
B) 22.0
C) 2.2×102
D) 22.02
E) 2.202×102

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
61
A carpenter must lay a floor that covers 1500 ft2. What is this area in cm2?
(1 foot = 12 inches and 1 inch = 2.54 cm)
A) 1.6 cm2
B) 49 cm2
C) 3.2×102 cm2
D) 4.6×104 cm2
E) 1.4×106 cm2
(1 foot = 12 inches and 1 inch = 2.54 cm)
A) 1.6 cm2
B) 49 cm2
C) 3.2×102 cm2
D) 4.6×104 cm2
E) 1.4×106 cm2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
62
A person weighs 83.2 kg. What is this person's weight in pounds?
(1)00 pound = 454 grams)
A) 0.183 pounds
B) 5.46 pounds
C) 37.8 pounds
D) 183 pounds
E) 3.78×107 pounds
(1)00 pound = 454 grams)
A) 0.183 pounds
B) 5.46 pounds
C) 37.8 pounds
D) 183 pounds
E) 3.78×107 pounds

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
63
The speed limit on the interstate in Virginia is 65 miles per hour. What is this speed in kilometers per second?
(1 km = 0.621 miles, 1 hour = 60 minutes and 1 minute = 60 seconds)
A) 0.029 km/sec
B) 34. km/sec
C) 40. km/sec
D) 55. km/sec
E) 1.0×102 km/sec
(1 km = 0.621 miles, 1 hour = 60 minutes and 1 minute = 60 seconds)
A) 0.029 km/sec
B) 34. km/sec
C) 40. km/sec
D) 55. km/sec
E) 1.0×102 km/sec

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
64
How many nanometers are present in 4.5 cm?
A) 4.5×10 - 9 nm
B) 4.5×10 - 7 nm
C) 4.5×106 nm
D) 4.5×107 nm
E) 4.5×1011 nm
A) 4.5×10 - 9 nm
B) 4.5×10 - 7 nm
C) 4.5×106 nm
D) 4.5×107 nm
E) 4.5×1011 nm

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
65
How many micrometers, m m, are present in 0.575 mm?
A) 5.75×10 - 10 m m
B) 5.75×10 - 4 m m
C) 5.75×101 m m
D) 5.75×102 m m
E) 5.75×108 m m
A) 5.75×10 - 10 m m
B) 5.75×10 - 4 m m
C) 5.75×101 m m
D) 5.75×102 m m
E) 5.75×108 m m

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
66
A car travels 28.0 miles per gallon of gasoline. How many kilometers per liter will it go?
(1 mile = 1.6093 km and 1 gallon = 3.7854 L)
A) 5.86×10 - 3 km/L
B) 4.60 km/L
C) 11.9 km/L
D) 65.9 km/L
E) 170 km/L
(1 mile = 1.6093 km and 1 gallon = 3.7854 L)
A) 5.86×10 - 3 km/L
B) 4.60 km/L
C) 11.9 km/L
D) 65.9 km/L
E) 170 km/L

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
67
The speed of light in a vacuum is 2.998×108 m/s. What is this speed given in cm/min?
A) 1.799×1013 cm/min
B) 1.799×1012 cm/min
C) 4.997×108 cm/min
D) 1.799×108 cm/min
E) 4.997×104 cm/min
A) 1.799×1013 cm/min
B) 1.799×1012 cm/min
C) 4.997×108 cm/min
D) 1.799×108 cm/min
E) 4.997×104 cm/min

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
68
Express a mass of 0.0220 kg in grams.
A) 22.0 g
B) 2.20×10 - 5 g
C) 2.20×10 - 3 g
D) 4.84×10 - 2 g
E) 0.0100 g
A) 22.0 g
B) 2.20×10 - 5 g
C) 2.20×10 - 3 g
D) 4.84×10 - 2 g
E) 0.0100 g

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
69
How many gallons are present in 43.7 Liters?
(1 gallon = 4 quarts and 1 quart = 0.946 L)
A) 10.3 gallons
B) 11.5 gallons
C) 46.2 gallons
D) 165 gallons
E) 185 gallons
(1 gallon = 4 quarts and 1 quart = 0.946 L)
A) 10.3 gallons
B) 11.5 gallons
C) 46.2 gallons
D) 165 gallons
E) 185 gallons

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
70
An object measures 1.92 meters in length. What is this object's length in feet?
(1 inch = 2.54 cm)
A) 9.07×10 - 2 ft
B) 5.85×10 - 1 ft
C) 6.30 ft
D) 4.06×101 ft
E) 9.07×102 ft
(1 inch = 2.54 cm)
A) 9.07×10 - 2 ft
B) 5.85×10 - 1 ft
C) 6.30 ft
D) 4.06×101 ft
E) 9.07×102 ft

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
71
The area of a US quarter is approximately 4.5×102 mm2. What is the area of a US quarter in square inches, (in2)?
(1 in = 2.54 cm)
A) 7.0×10 - 1 in2
B) 1.8×101 in2
C) 2.9×105 in2
D) 1.8×10 - 1 in2
E) 1.1×104 in2
(1 in = 2.54 cm)
A) 7.0×10 - 1 in2
B) 1.8×101 in2
C) 2.9×105 in2
D) 1.8×10 - 1 in2
E) 1.1×104 in2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
72
The smallest bone in the human body, which is in the ear, has a mass of 0.0030 grams. What is this mass in pounds?
(1 pound = 454 grams)
A) 6.6×10 - 6 pounds
B) 3.0×10 - 3 pounds
C) 0.73 pounds
D) 1.4 pounds
E) 1.5×105 pounds
(1 pound = 454 grams)
A) 6.6×10 - 6 pounds
B) 3.0×10 - 3 pounds
C) 0.73 pounds
D) 1.4 pounds
E) 1.5×105 pounds

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
73
Analysis shows the presence of 203 m g of cholesterol in a sample of blood. How many grams of cholesterol are present in this blood sample?
A) 2.03×10 - 6 g
B) 2.03×10 - 4 g
C) 2.03×102 g
D) 2.03×106 g
E) 2.03×108 g
A) 2.03×10 - 6 g
B) 2.03×10 - 4 g
C) 2.03×102 g
D) 2.03×106 g
E) 2.03×108 g

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
74
The atomic radius of a beryllium atom is 111 pm. Express this distance in inches.
A) 2.67×10 - 8 inch
B) 4.37×10 - 9 inch
C) 2.42×10 - 13 inch
D) 4.37×10 - 13 inch
E) 43.7 inch
A) 2.67×10 - 8 inch
B) 4.37×10 - 9 inch
C) 2.42×10 - 13 inch
D) 4.37×10 - 13 inch
E) 43.7 inch

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
75
The diameter of a US Quarter is approximately 2.35 cm. What is the diameter of a quarter expressed in km and scientific notation ?
A) 2.35×10 - 5 km
B) 2.35×105 km
C) 2.35×10 - 2 km
D) 23.5×10 - 4 km
E) 2.35 km
A) 2.35×10 - 5 km
B) 2.35×105 km
C) 2.35×10 - 2 km
D) 23.5×10 - 4 km
E) 2.35 km

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
76
Express 15 inches in cm.
A) 2.5 cm
B) 5.9 cm
C) 38 cm
D) 38.1 cm
E) 38.10 cm
A) 2.5 cm
B) 5.9 cm
C) 38 cm
D) 38.1 cm
E) 38.10 cm

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
77
The speed limit is 70 miles per hour on some stretches of the interstate. What is this same speed in meters per second, (m/s)?
(1 mile = 1.60 km)
A) 1.9 m/s
B) 31 m/s
C) 1.6×102 m/s
D) 1.1×105 m/s
E) 1.1×102 m/s
(1 mile = 1.60 km)
A) 1.9 m/s
B) 31 m/s
C) 1.6×102 m/s
D) 1.1×105 m/s
E) 1.1×102 m/s

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
78
The area of a telescope lens is 5773 mm2. What is this area given in square feet, ft2?
(1 inch = 2.54 cm)
A) 6.214×10 - 2 ft2
B) 8.948 ft2
C) 1.894×101 ft2
D) 1.288×103 ft2
E) 5.363×104 ft2
(1 inch = 2.54 cm)
A) 6.214×10 - 2 ft2
B) 8.948 ft2
C) 1.894×101 ft2
D) 1.288×103 ft2
E) 5.363×104 ft2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
79
In a new measuring system created by your instructor, 2.16 geb = 1.02 red. How many geb is 7.4 red?
A) 3.5 geb
B) 3.49 geb
C) 15.7 geb
D) 16 geb
E) 1.57 geb
A) 3.5 geb
B) 3.49 geb
C) 15.7 geb
D) 16 geb
E) 1.57 geb

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
80
What is the speed of an automobile given in miles per second (miles/sec) that is traveling 80. kilometer per hour (km/hour)?
(1 km = 0.621 miles; 1 hour = 60 min; 1 min = 60 sec)
A) 1.4×10 - 2 miles/s
B) 3.6×10 - 2 miles/s
C) 50 miles/s
D) 1.3×102 miles/s
E) 1.8×105 miles/s
(1 km = 0.621 miles; 1 hour = 60 min; 1 min = 60 sec)
A) 1.4×10 - 2 miles/s
B) 3.6×10 - 2 miles/s
C) 50 miles/s
D) 1.3×102 miles/s
E) 1.8×105 miles/s

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck


