Deck 7: Electronic Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/86
Play
Full screen (f)
Deck 7: Electronic Structure
1
What is the wavelength of light with a frequency of 5.2×1014 Hz?
A) 1.7×106 m
B) 1.9×10 - 7 m
C) 5.8×10 - 7 m
D) 3.8×10 - 5 m
E) none of these
A) 1.7×106 m
B) 1.9×10 - 7 m
C) 5.8×10 - 7 m
D) 3.8×10 - 5 m
E) none of these
5.8×10 - 7 m
2
The threshold frequency for the photoelectric effect of a particular metal is 3.0×1014 sec - 1. When this metal is illuminated by light with a frequency of 2.0×1014 sec - 1, which of the following is true?
A) The kinetic energy of the emitted electrons is greater than those emitted by light at the threshold frequency.
B) The number of electrons emitted is greater than that emitted by light at the threshold frequency.
C) The light energy absorbed by the metal decreases the distance between atoms.
D) No electrons will be emitted due to the photoelectric effect.
E) None of these are true.
A) The kinetic energy of the emitted electrons is greater than those emitted by light at the threshold frequency.
B) The number of electrons emitted is greater than that emitted by light at the threshold frequency.
C) The light energy absorbed by the metal decreases the distance between atoms.
D) No electrons will be emitted due to the photoelectric effect.
E) None of these are true.
No electrons will be emitted due to the photoelectric effect.
3
The energy of a photon of electromagnetic radiation with a wavelength of 1.0 nm is
A) 9.2×10 - 13 J
B) 5.0×10 - 16 J
C) 2.0×10 - 16 J
D) 1.1×10 - 14 J
E) none of these
A) 9.2×10 - 13 J
B) 5.0×10 - 16 J
C) 2.0×10 - 16 J
D) 1.1×10 - 14 J
E) none of these
2.0×10 - 16 J
4
Calculate the wavelength of light emitted when an electron in a hydrogen atom falls from the n = 4 orbit to the n = 1 orbit (Rh = 1.1×107 m - 1).
A) 9.7×10 - 8 m
B) 6.5×10 - 7 m
C) 3.6×10 - 7 m
D) 6.4×10 - 6 m
E) none of these
A) 9.7×10 - 8 m
B) 6.5×10 - 7 m
C) 3.6×10 - 7 m
D) 6.4×10 - 6 m
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
5
An argon ion laser emits light at 489 nm. What is the frequency of this radiation?
A) 147 sec - 1
B) 6.13×105 sec - 1
C) 1.47×1011 sec - 1
D) 6.13×1014 sec - 1
E) None of these
A) 147 sec - 1
B) 6.13×105 sec - 1
C) 1.47×1011 sec - 1
D) 6.13×1014 sec - 1
E) None of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
6
One type of sunburn occurs on exposure to UV light of wavelength equal to 325 nm. What is the frequency given in sec - 1 for this wavelength?
A) 1.08×10 - 15 sec - 1
B) 9.23×10 - 4 sec - 1
C) 9.75×101 sec - 1
D) 9.23×105 sec - 1
E) 9.23×1014 sec - 1
A) 1.08×10 - 15 sec - 1
B) 9.23×10 - 4 sec - 1
C) 9.75×101 sec - 1
D) 9.23×105 sec - 1
E) 9.23×1014 sec - 1

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
7
What is the energy of a photon of light that has a frequency of 2.3×1014 s - 1 (h = 6.6×10 - 34 J × s)?
A) 2.8×10 - 18 J
B) 1.5×10 - 19 J
C) 2.9×10 - 48 J
D) 6.4×10 - 22 J
E) none of these
A) 2.8×10 - 18 J
B) 1.5×10 - 19 J
C) 2.9×10 - 48 J
D) 6.4×10 - 22 J
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
8
Calculate the frequency of light whose wavelength is 4.2×10 - 7 meters.
A) 6.2×1013 s - 1
B) 7.1×1014 s - 1
C) 8.3×1013 s - 1
D) 1.8×1014 s - 1
E) none of these
A) 6.2×1013 s - 1
B) 7.1×1014 s - 1
C) 8.3×1013 s - 1
D) 1.8×1014 s - 1
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
9
Calculate the frequency of light whose wavelength is 711 nm.
A) 2.80×10 - 19 s - 1
B) 4.22×1014 s - 1
C) 4.22×105 s - 1
D) 2.10×108 s - 1
E) none of these
A) 2.80×10 - 19 s - 1
B) 4.22×1014 s - 1
C) 4.22×105 s - 1
D) 2.10×108 s - 1
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
10
The energy of a photon of electromagnetic radiation with a frequency of 1.5×1014 Hz is
A) 6.6×10 - 34 J
B) 9.9×10 - 20 J
C) 9.9×10 - 17 J
D) 1.1×10 - 14 J
E) none of these
A) 6.6×10 - 34 J
B) 9.9×10 - 20 J
C) 9.9×10 - 17 J
D) 1.1×10 - 14 J
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
11
The distance between two adjacent peaks of a wave is known as the ____ of the wave.
A) frequency
B) wave amplitude
C) wavelength
D) velocity
E) none of these
A) frequency
B) wave amplitude
C) wavelength
D) velocity
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
12
A certain blue light has a wavelength oF453 nm ( l = 453 nm). What is the energy (in Joules) of one photon of this blue light with this wavelength?
A) 9.97×10 - 49 J
B) 2.99×10 - 40 J
C) 1.46×10 - 27 J
D) 4.37×10 - 19 J
E) 6.86×1026 J
A) 9.97×10 - 49 J
B) 2.99×10 - 40 J
C) 1.46×10 - 27 J
D) 4.37×10 - 19 J
E) 6.86×1026 J

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
13
Calculate the frequency of light whose wavelength is 5.0×10 - 6 meters.
A) 6.0×1013 s - 1
B) 2.0×105 s - 1
C) 3.0×108 s - 1
D) 4.0×10 - 20 s - 1
E) none of these
A) 6.0×1013 s - 1
B) 2.0×105 s - 1
C) 3.0×108 s - 1
D) 4.0×10 - 20 s - 1
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
14
What is the wavelength of light with a frequency of 8.2×1013 s - 1?
A) 2.8×10 - 5 m
B) 7.2×10 - 7 m
C) 4.8×10 - 7 m
D) 3.7×10 - 6 m
E) none of these
A) 2.8×10 - 5 m
B) 7.2×10 - 7 m
C) 4.8×10 - 7 m
D) 3.7×10 - 6 m
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
15
If waves are hitting the beach every 2.0 seconds and are 12 m apart, what is the velocity of the waves?
A) 6.0 m/s
B) 24 m/s
C) 18 m/s
D) 2.0 m/s
E) none of these
A) 6.0 m/s
B) 24 m/s
C) 18 m/s
D) 2.0 m/s
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
16
How much energy does a single photon of an argon ion laser emit if the wavelength of this photon is 489 nm?
A) 4.06×10 - 19 J
B) 9.74×10 - 23 J
C) 4.06×10 - 28 J
D) 9.74×10 - 32 J
E) None of these
A) 4.06×10 - 19 J
B) 9.74×10 - 23 J
C) 4.06×10 - 28 J
D) 9.74×10 - 32 J
E) None of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
17
Which equation below properly relates energy (E) to wavelength ( l ) given h = Plank's constant, c = speed of light, and n = frequency?
A) E = h l
B)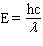
C)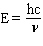
D)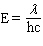
E) none of these
A) E = h l
B)
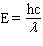
C)
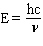
D)
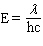
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
18
The number of wave crests that pass a fixed point each second is known as the ____ of the wave.
A) frequency
B) wave amplitude
C) wavelength
D) velocity
E) none of these
A) frequency
B) wave amplitude
C) wavelength
D) velocity
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
19
What is the energy of one photon of radiation with a wavelength of 325 nm?
A) 7.13×10 - 49 J
B) 6.09×10 - 37 J
C) 6.43×10 - 32 J
D) 2.15×10 - 31 J
E) 6.09×10 - 19 J
A) 7.13×10 - 49 J
B) 6.09×10 - 37 J
C) 6.43×10 - 32 J
D) 2.15×10 - 31 J
E) 6.09×10 - 19 J

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
20
The frequency of a photon of electromagnetic radiation with an energy of 7.3×10 - 18 J is
A) 2.7×10 - 8 s - 1
B) 9.9×1019 s - 1
C) 2.0×1016 s - 1
D) 1.1×1016 s - 1
E) none of these
A) 2.7×10 - 8 s - 1
B) 9.9×1019 s - 1
C) 2.0×1016 s - 1
D) 1.1×1016 s - 1
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
21
What quantum number(s) provide(s) information with respect to the shape of an atomic orbital?
A) Principal quantum number, "n"
B) Angular Momentum quantum number, "l"
C) Magnetic quantum number, "ml"
D) Electron spin quantum number, "ms"
E) Principle and Angular Momentum quantum numbers
A) Principal quantum number, "n"
B) Angular Momentum quantum number, "l"
C) Magnetic quantum number, "ml"
D) Electron spin quantum number, "ms"
E) Principle and Angular Momentum quantum numbers

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
22
What is the wavelength of an electron (mass = 9.11×10 - 31 kg) moving with a velocity of 60 m/s (h = 6.6×10 - 34 J × s)?
A) 2.8×10 - 5 m
B) 3.6×10 - 7 m
C) 1.2×10 - 5 m
D) 4.2×10 - 7 m
E) none of these
A) 2.8×10 - 5 m
B) 3.6×10 - 7 m
C) 1.2×10 - 5 m
D) 4.2×10 - 7 m
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
23
Calculate the wavelength of light emitted when an electron in a He+ ion goes from the n = 5 shell to the n = 2 shell.
A) 2.30×106 m
B) 4.34×10 - 7 m
C) 1.08×10 - 7 m
D) 1.52×10 - 7 m
E) none of these
A) 2.30×106 m
B) 4.34×10 - 7 m
C) 1.08×10 - 7 m
D) 1.52×10 - 7 m
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
24
Three sets of quantum numbers are given below. Choose the best answer.
I. n = 3,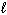 = 3,
= 3, 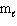 = 0, m s = 1 / 2
= 0, m s = 1 / 2
II. n = 2,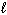 = 1,
= 1, 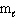 = 1, m s = 1 / 2
= 1, m s = 1 / 2
III. n = 4,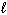 = 2,
= 2, 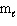 = 3, m s = - 1 / 2
= 3, m s = - 1 / 2
A) I and II are allowed sets, III is not
B) I and III are allowed sets, II is not
C) only I is allowed
D) only II is allowed
E) none of these are allowed
I. n = 3,
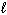 = 3,
= 3, 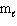 = 0, m s = 1 / 2
= 0, m s = 1 / 2II. n = 2,
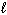 = 1,
= 1, 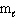 = 1, m s = 1 / 2
= 1, m s = 1 / 2III. n = 4,
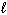 = 2,
= 2, 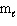 = 3, m s = - 1 / 2
= 3, m s = - 1 / 2A) I and II are allowed sets, III is not
B) I and III are allowed sets, II is not
C) only I is allowed
D) only II is allowed
E) none of these are allowed

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
25
From the modern day Quantum theory of the atom, which theory postulates that the mass of the electron and its exact position cannot be deduced simultaneously and that we must be satisfied with the probability of finding the electron?
A) Pauli Exclusion Principle
B) Heisenberg uncertainty principle
C) Einstein's theory of relativity
D) Light as a particle and a wave
E) Bohr's model of the Hydrogen atom
A) Pauli Exclusion Principle
B) Heisenberg uncertainty principle
C) Einstein's theory of relativity
D) Light as a particle and a wave
E) Bohr's model of the Hydrogen atom

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
26
According to the Bohr model for a hydrogen atom, the lines on the hydrogen emission spectrum are related to what type of energy change?
A) Electronic transitions from a higher energy state to a lower energy state.
B) Electronic transitions from a lower to a higher energy state.
C) The amount of energy released during high speed collisions of hydrogen molecules.
D) The amount of energy released when hydrogen molecules are split into hydrogen atoms during an electrical discharge in a vacuum tube.
E) None of these.
A) Electronic transitions from a higher energy state to a lower energy state.
B) Electronic transitions from a lower to a higher energy state.
C) The amount of energy released during high speed collisions of hydrogen molecules.
D) The amount of energy released when hydrogen molecules are split into hydrogen atoms during an electrical discharge in a vacuum tube.
E) None of these.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
27
What quantum number(s) provide(s) information with respect to the spatial orientation of an atomic orbital?
A) Principal quantum number, "n"
B) Angular Momentum quantum number, "l"
C) Magnetic quantum number, "ml"
D) Electron spin quantum number, "ms"
E) Principle and Angular Momentum quantum numbers
A) Principal quantum number, "n"
B) Angular Momentum quantum number, "l"
C) Magnetic quantum number, "ml"
D) Electron spin quantum number, "ms"
E) Principle and Angular Momentum quantum numbers

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
28
What quantum number(s) provide(s) information with respect to the size of an atomic orbital?
A) Principal quantum number, "n"
B) Angular Momentum quantum number, "l"
C) Magnetic quantum number, "ml"
D) Electron spin quantum number, "ms"
E) Principle and Angular Momentum quantum numbers
A) Principal quantum number, "n"
B) Angular Momentum quantum number, "l"
C) Magnetic quantum number, "ml"
D) Electron spin quantum number, "ms"
E) Principle and Angular Momentum quantum numbers

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
29
What quantum number(s) is(are) required to completely describe an orbital , the region in space in which an electron has a high probability of being found?
I. Principal quantum number
II. Angular Momentum quantum number
III. Magnetic quantum number
A) I only
B) I and II
C) I and III
D) II and III
E) I, II and III
I. Principal quantum number
II. Angular Momentum quantum number
III. Magnetic quantum number
A) I only
B) I and II
C) I and III
D) II and III
E) I, II and III

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
30
The energy of the electron in the hydrogen atom depends on the quantum number(s)
A) n only
B) n and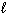
C)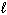 and
and 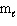
D)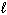 only
only
E) n,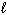 and
and 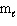
A) n only
B) n and
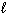
C)
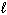 and
and 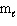
D)
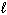 only
onlyE) n,
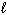 and
and 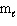

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
31
Three sets of quantum numbers are listed below. Pick the best answer.
I. n = 2,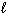 = 2,
= 2, 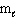 = 2, m s = 1 / 2
= 2, m s = 1 / 2
II. n = 4,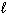 = 0,
= 0, 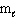 = 0, m s = 1 / 2
= 0, m s = 1 / 2
III. n = 3,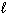 = 2,
= 2, 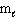 = 3, m s = 1 / 2
= 3, m s = 1 / 2
A) I and II are allowed sets, III is not
B) II and III are allowed sets, I is not
C) all 3 are allowed sets
D) only II is an allowed set
E) all 3 are not allowed sets
I. n = 2,
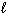 = 2,
= 2, 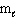 = 2, m s = 1 / 2
= 2, m s = 1 / 2II. n = 4,
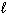 = 0,
= 0, 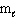 = 0, m s = 1 / 2
= 0, m s = 1 / 2III. n = 3,
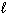 = 2,
= 2, 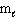 = 3, m s = 1 / 2
= 3, m s = 1 / 2A) I and II are allowed sets, III is not
B) II and III are allowed sets, I is not
C) all 3 are allowed sets
D) only II is an allowed set
E) all 3 are not allowed sets

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
32
What quantum number(s) is(are) required to completely describe a subshell , a set of orbitals with the same energy and shape?
I. Principal quantum number
II. Angular Momentum quantum number
III. Magnetic quantum number
A) I only
B) I and II
C) I and III
D) II and III
E) I, II and III
I. Principal quantum number
II. Angular Momentum quantum number
III. Magnetic quantum number
A) I only
B) I and II
C) I and III
D) II and III
E) I, II and III

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
33
The quantum number l for an electron in an atom is 2. This electron is definitely located in:
A) the first principal shell
B) the second principal shell
C) an s subshell
D) a p subshell
E) a d subshell
A) the first principal shell
B) the second principal shell
C) an s subshell
D) a p subshell
E) a d subshell

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
34
Calculate the wavelength of light emitted when an electron in the hydrogen atom goes from the n = 4 shell to the n = 2 shell.
A) 2.06×106 m
B) 4.86×10 - 7 m
C) 3.65×10 - 7 m
D) 4.09×10 - 19 m
E) none of these
A) 2.06×106 m
B) 4.86×10 - 7 m
C) 3.65×10 - 7 m
D) 4.09×10 - 19 m
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
35
From the restrictions on the three quantum numbers that define an orbital, which set of three quantum numbers listed below is forbidden ?
A) n = 3,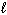 = 3,
= 3, 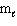 = 3
= 3
B) n = 3,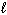 = 2,
= 2, 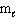 = - 2
= - 2
C) n = 3,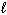 = 2,
= 2, 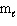 = +2
= +2
D) n = 3,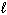 = 1,
= 1, 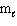 = +1
= +1
E) n = 3,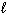 = 0,
= 0, 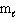 = 0
= 0
A) n = 3,
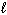 = 3,
= 3, 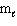 = 3
= 3B) n = 3,
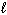 = 2,
= 2, 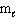 = - 2
= - 2C) n = 3,
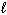 = 2,
= 2, 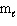 = +2
= +2D) n = 3,
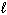 = 1,
= 1, 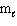 = +1
= +1E) n = 3,
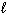 = 0,
= 0, 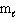 = 0
= 0
Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
36
Given the three statements below, pick the best answer.
I. c = ln
II. E = h n
III. heating gaseous Li atoms yields white light
A) II and III are true, I is false
B) I and II are true, III is false
C) I and III are true, II is false
D) all three are true
E) only I is true
I. c = ln
II. E = h n
III. heating gaseous Li atoms yields white light
A) II and III are true, I is false
B) I and II are true, III is false
C) I and III are true, II is false
D) all three are true
E) only I is true

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
37
Only one set of quantum numbers listed below is a correct set. Which one?
(n, l , m l , ms)
A) 3, 2, 0, 1/2
B) 2, 2, - 2, 1/2
C) 3, 3, - 3, 1/2
D) 4, 4, - 3, 1/2
E) none of these
(n, l , m l , ms)
A) 3, 2, 0, 1/2
B) 2, 2, - 2, 1/2
C) 3, 3, - 3, 1/2
D) 4, 4, - 3, 1/2
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the frequency of light emitted when an electron in a hydrogen atom goes from the n = 5 shell to the n = 2 shell.
A) 6.9×1014 s - 1
B) 8.2×1013 s - 1
C) 7.8×1014 s - 1
D) 1.2×1014 s - 1
E) none of these
A) 6.9×1014 s - 1
B) 8.2×1013 s - 1
C) 7.8×1014 s - 1
D) 1.2×1014 s - 1
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
39
Given the three statements below, which answer is best?
I. Although the wavelength of light and the frequency of light can change, the energy remains constant.
II. An important consequence of quantum theory is that the energy of various electronic states for atoms are at fixed discrete levels.
III. The energy of an electronic state for a hydrogen atom is dependent only on the principal quantum number n.
A) I and II are true, III is false
B) II and III are true, I is false
C) I and III are true, II is false
D) all are true
E) all are false
I. Although the wavelength of light and the frequency of light can change, the energy remains constant.
II. An important consequence of quantum theory is that the energy of various electronic states for atoms are at fixed discrete levels.
III. The energy of an electronic state for a hydrogen atom is dependent only on the principal quantum number n.
A) I and II are true, III is false
B) II and III are true, I is false
C) I and III are true, II is false
D) all are true
E) all are false

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
40
Scientists of the 19th Century found that the equation, 1/ l = RH(1/n21 - 1/n22) (where RH = 1.097×107 m - 1, and n1 and n2 are whole numbers) predicts the wavelength of the lines in the emission spectrum of the H atom. This equation is properly referred to as:
A) a theory
B) a postulate
C) a law
D) a hypothesis
E) none of these
A) a theory
B) a postulate
C) a law
D) a hypothesis
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
41
Three sets of quantum numbers are listed below. Pick the best answer.
I. n = 3,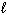 = 3,
= 3, 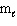 = 2
= 2
II. n = 4,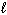 = 2,
= 2, 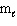 = 0
= 0
III. n = 1,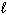 = 0,
= 0, 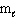 = 0
= 0
A) I and II are allowed sets, III is not
B) only III is an allowed set
C) II and III are allowed sets, I is not
D) all three sets are allowed
E) only II is an allowed set
I. n = 3,
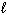 = 3,
= 3, 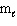 = 2
= 2II. n = 4,
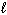 = 2,
= 2, 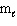 = 0
= 0III. n = 1,
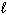 = 0,
= 0, 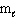 = 0
= 0A) I and II are allowed sets, III is not
B) only III is an allowed set
C) II and III are allowed sets, I is not
D) all three sets are allowed
E) only II is an allowed set

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
42
Which set(s) of three quantum numbers is(are) permissible?
I. n = 2,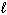 = 1, and
= 1, and 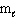 = 1
= 1
II. n = 4,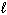 = 2, and
= 2, and 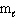 = - 2
= - 2
III. n = 3,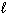 = 3, and
= 3, and 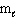 = 0
= 0
A) I only
B) II only
C) III only
D) I and II
E) All of these.
I. n = 2,
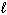 = 1, and
= 1, and 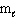 = 1
= 1II. n = 4,
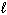 = 2, and
= 2, and 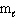 = - 2
= - 2III. n = 3,
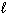 = 3, and
= 3, and 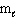 = 0
= 0A) I only
B) II only
C) III only
D) I and II
E) All of these.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
43
How many possible orientations are allowed when 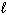 = 0?
= 0?
(s-type sublevel; How many possible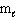 values are possible?)
values are possible?)
A) 1
B) 2
C) 3
D) 5
E) 7
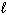 = 0?
= 0?(s-type sublevel; How many possible
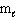 values are possible?)
values are possible?)A) 1
B) 2
C) 3
D) 5
E) 7

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
44
Exhibit 7-1 Consider the shapes listed below to answer the following question(s):
I.
II.
III.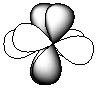
IV.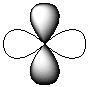
V.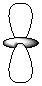
Refer to Exhibit 7-1. Which of the shapes would represent one of seven possible 4f atomic orbitals?
A) I
B) II
C) III
D) IV
E) V
I.

II.

III.

IV.

V.

Refer to Exhibit 7-1. Which of the shapes would represent one of seven possible 4f atomic orbitals?
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
45
From the restrictions on the three quantum numbers that define an orbital, which set of three quantum numbers listed below is forbidden?
A) n = 4,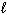 = 3,
= 3, 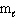 = - 2
= - 2
B) n = 4,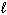 = 2,
= 2, 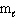 = - 2
= - 2
C) n = 4,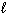 = 3,
= 3, 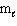 = 3
= 3
D) n = 4,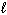 = 4,
= 4, 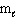 = - 2
= - 2
E) n = 4,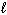 = 1,
= 1, 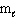 = 1
= 1
A) n = 4,
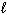 = 3,
= 3, 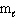 = - 2
= - 2B) n = 4,
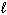 = 2,
= 2, 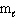 = - 2
= - 2C) n = 4,
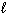 = 3,
= 3, 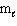 = 3
= 3D) n = 4,
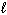 = 4,
= 4, 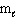 = - 2
= - 2E) n = 4,
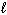 = 1,
= 1, 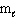 = 1
= 1
Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
46
How many possible orientations are allowed when 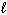 = 2?
= 2?
(d-type sublevel; How many possible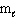 values are possible?)
values are possible?)
A) 1
B) 2
C) 3
D) 5
E) 7
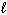 = 2?
= 2?(d-type sublevel; How many possible
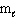 values are possible?)
values are possible?)A) 1
B) 2
C) 3
D) 5
E) 7

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
47
The quantum number 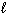 for a 3d electron is:
for a 3d electron is:
A) 1
B) 2
C) 3
D) 4
E) none of these
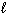 for a 3d electron is:
for a 3d electron is:A) 1
B) 2
C) 3
D) 4
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
48
How many possible orientations are allowed when 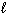 = 1?
= 1?
(p-type sublevel; How many possible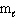 values are possible?)
values are possible?)
A) 1
B) 2
C) 3
D) 5
E) 7
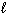 = 1?
= 1?(p-type sublevel; How many possible
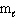 values are possible?)
values are possible?)A) 1
B) 2
C) 3
D) 5
E) 7

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
49
How many electrons can be placed in the subshell described by the quantum numbers n = 3, 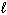 = 1?
= 1?
A) 1
B) 2
C) 10
D) 6
E) none of these
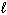 = 1?
= 1?A) 1
B) 2
C) 10
D) 6
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
50
What are the possible 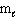 values for 3p electrons?
values for 3p electrons?
A) 0, 1, 2
B) 1, 2, 3
C) - 1, 0, +1
D) - 2, 0, +1, +2
E) none of these
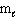 values for 3p electrons?
values for 3p electrons?A) 0, 1, 2
B) 1, 2, 3
C) - 1, 0, +1
D) - 2, 0, +1, +2
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
51
Exhibit 7-1 Consider the shapes listed below to answer the following question(s):
I.
II.
III.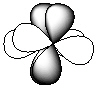
IV.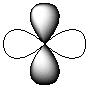
V.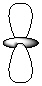
Refer to Exhibit 7-1. Which of the shapes would represent one of three possible 2p atomic orbitals?
A) I
B) II
C) III
D) IV
E) V
I.

II.

III.

IV.

V.

Refer to Exhibit 7-1. Which of the shapes would represent one of three possible 2p atomic orbitals?
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
52
How many possible orientations are allowed when 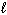 = 3?
= 3?
(f-type sublevel; How many possible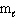 values are possible?)
values are possible?)
A) 1
B) 2
C) 3
D) 5
E) 7
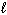 = 3?
= 3?(f-type sublevel; How many possible
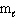 values are possible?)
values are possible?)A) 1
B) 2
C) 3
D) 5
E) 7

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
53
How many electrons can be placed in the subshell described by the quantum numbers n = 2, 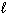 = 0?
= 0?
A) none
B) 2
C) 6
D) 10
E) none of these
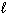 = 0?
= 0?A) none
B) 2
C) 6
D) 10
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
54
Which full set of four quantum numbers would be expected for the highest energy electron for a potassium atom?
A) n = 4,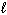 = 0,
= 0, 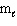 = 0, m s = +1/2
= 0, m s = +1/2
B) n = 4,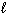 = 1,
= 1, 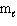 = 1, m s = +1/2
= 1, m s = +1/2
C) n = 4,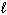 = 2,
= 2, 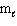 = 2, m s = +1/2
= 2, m s = +1/2
D) n = 3,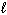 = 1,
= 1, 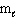 = 1, m s = +1/2
= 1, m s = +1/2
E) n = 3,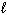 = 2,
= 2, 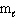 = 2, m s = +1/2
= 2, m s = +1/2
A) n = 4,
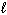 = 0,
= 0, 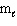 = 0, m s = +1/2
= 0, m s = +1/2B) n = 4,
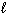 = 1,
= 1, 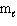 = 1, m s = +1/2
= 1, m s = +1/2C) n = 4,
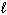 = 2,
= 2, 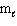 = 2, m s = +1/2
= 2, m s = +1/2D) n = 3,
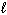 = 1,
= 1, 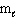 = 1, m s = +1/2
= 1, m s = +1/2E) n = 3,
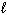 = 2,
= 2, 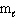 = 2, m s = +1/2
= 2, m s = +1/2
Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
55
The possible values of the magnetic quantum number, 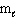 , of a 3d electron are:
, of a 3d electron are:
A) 1, 2, 3
B) 0, 1, 2
C) - 2, - 1, 0, 1, 2
D) - 1, 0, 1
E) none of these
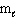 , of a 3d electron are:
, of a 3d electron are:A) 1, 2, 3
B) 0, 1, 2
C) - 2, - 1, 0, 1, 2
D) - 1, 0, 1
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
56
A subshell is described by assigning values to the quantum numbers
A) n only
B) n,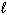 , and
, and 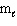
C) n and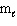
D) n and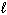
E)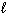 only
only
A) n only
B) n,
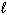 , and
, and 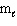
C) n and
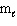
D) n and
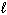
E)
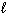 only
only
Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
57
What is the order for filling the following subshells with electrons?
4d, 4f, 5s, 5p, 6s
A) 4d, 4f, 5s, 5p, 6s
B) 5s, 6s, 5p, 4d, 4f
C) 5s, 4d, 5p, 6s, 4f
D) 4d, 4f, 5p, 5s, 6s
E) 6s, 5p, 5s, 4f, 4d
4d, 4f, 5s, 5p, 6s
A) 4d, 4f, 5s, 5p, 6s
B) 5s, 6s, 5p, 4d, 4f
C) 5s, 4d, 5p, 6s, 4f
D) 4d, 4f, 5p, 5s, 6s
E) 6s, 5p, 5s, 4f, 4d

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
58
What is the order for filling the following atomic subshells?
(Lowest energy to highest energy) 4f, 5p, 5d, 6s
A) 4f < 5p < 5d < 6s
B) 6s < 5p < 5d < 4f
C) 6s < 5d < 5p < 4f
D) 4f < 5d < 5p < 6s
E) 5p < 6s < 4f < 5d
(Lowest energy to highest energy) 4f, 5p, 5d, 6s
A) 4f < 5p < 5d < 6s
B) 6s < 5p < 5d < 4f
C) 6s < 5d < 5p < 4f
D) 4f < 5d < 5p < 6s
E) 5p < 6s < 4f < 5d

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
59
The possible values of the magnetic quantum number, 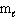 , of a 2p electron are:
, of a 2p electron are:
A) - 1, 0, 1
B) - 2, - 1, 0, 1, 2
C) 0, 1, 2
D) 1, 2, 3
E) none of these
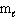 , of a 2p electron are:
, of a 2p electron are:A) - 1, 0, 1
B) - 2, - 1, 0, 1, 2
C) 0, 1, 2
D) 1, 2, 3
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
60
What is the maximum number of electrons that can occupy a 5d sublevel?
A) 2
B) 5
C) 6
D) 10
E) 14
A) 2
B) 5
C) 6
D) 10
E) 14

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
61
Which orbital diagram for the ground state electronic configuration of a Nitrogen atom is correct?
A)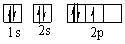
B)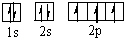
C)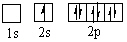
D)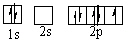
E)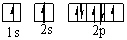
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
62
What is the correct electronic configuration for Zirconium (Z = 40)?
A) 1s22s22p63s23p63d104s24p64d25s2
B) 1s22s22p63s23p64s23d104p65s24d2
C) 1s21p61d10 2s22p62d103s23p4
D) 1s22s23s24s25s22p63p64p63d104d2
E) 1d21f61p101s142d22f6
A) 1s22s22p63s23p63d104s24p64d25s2
B) 1s22s22p63s23p64s23d104p65s24d2
C) 1s21p61d10 2s22p62d103s23p4
D) 1s22s23s24s25s22p63p64p63d104d2
E) 1d21f61p101s142d22f6

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
63
What is the ground state electron configuration of fluorine?
A) 1s22s22p7
B) 1s22s22p5
C) 1s22s22p3
D) 1s22s23s5
E) none of these
A) 1s22s22p7
B) 1s22s22p5
C) 1s22s22p3
D) 1s22s23s5
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
64
How many orbitals are present in a 3d subshell?
A) 1 orbital
B) 3 orbitals
C) 5 orbitals
D) 7 orbitals
E) 9 orbitals
A) 1 orbital
B) 3 orbitals
C) 5 orbitals
D) 7 orbitals
E) 9 orbitals

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
65
The element with a ground state electron configuration of 1s22s22p6 is
A) B
B) C
C) O
D) F
E) Ne
A) B
B) C
C) O
D) F
E) Ne

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following orbital diagrams for the ground state electronic configuration for a phosphorus atom is correct?
A)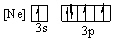
B)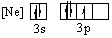
C)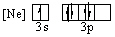
D)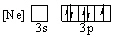
E)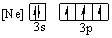
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
67
Which orbital diagram for the ground state electronic configuration of an oxygen atom is correct?
A)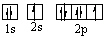
B)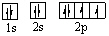
C)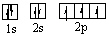
D)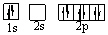
E)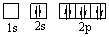
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
68
The Pauli exclusion principle:
A) allows only two electrons to occupy a given orbital.
B) requires that the spins of unpaired electrons be parallel.
C) limits the knowledge we can have concerning the simultaneous position and momentum of an electron.
D) gives the allowed energies of an electron in a hydrogen atom.
E) choices a-d are all incorrect.
A) allows only two electrons to occupy a given orbital.
B) requires that the spins of unpaired electrons be parallel.
C) limits the knowledge we can have concerning the simultaneous position and momentum of an electron.
D) gives the allowed energies of an electron in a hydrogen atom.
E) choices a-d are all incorrect.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
69
What is the correct electronic configuration for the element Manganese (Z = 25)?
A) [Ar]3d7
B) 1s22s22p63s23p63d54s2
C) 1s22s22p63s23p63d7
D) 1s22s22p63s23p64s23d5
E) [Kr]3d7
A) [Ar]3d7
B) 1s22s22p63s23p63d54s2
C) 1s22s22p63s23p63d7
D) 1s22s22p63s23p64s23d5
E) [Kr]3d7

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
70
What is the ground state electron configuration of boron?
A) 1s22s22p3
B) 1s22s22p2
C) 1s22s22p1
D) 1s22s23s1
E) none of these
A) 1s22s22p3
B) 1s22s22p2
C) 1s22s22p1
D) 1s22s23s1
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
71
What is the ground state electron configuration of oxygen?
A) 1s22s22p4
B) 1s22s22p2
C) 1s22s22p3
D) 1s22s23s4
E) none of these
A) 1s22s22p4
B) 1s22s22p2
C) 1s22s22p3
D) 1s22s23s4
E) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
72
Which atomic subshell can hold up to 14 electrons?
A) 3d
B) 4s
C) 2p
D) 5f
E) 3p
A) 3d
B) 4s
C) 2p
D) 5f
E) 3p

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
73
What is the correct electronic configuration for Nickel (Z = 28)?
A) [Ar]3d10
B) 1s22s22p63s23p63d84s2
C) 1s22s22p63s23p63d10
D) 1s22s22p63s23p64s23d8
E) None of these
A) [Ar]3d10
B) 1s22s22p63s23p63d84s2
C) 1s22s22p63s23p63d10
D) 1s22s22p63s23p64s23d8
E) None of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
74
What is the maximum number of electrons that can occupy a 5f subshell?
A) 2
B) 5
C) 6
D) 10
E) 14
A) 2
B) 5
C) 6
D) 10
E) 14

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
75
Which subshell can hold up to a total of ten electrons?
A) s-subshell
B) p-subshell
C) d-subshell
D) f-subshell
E) a and b
A) s-subshell
B) p-subshell
C) d-subshell
D) f-subshell
E) a and b

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
76
What is the correct ground state electronic configuration of Lead (Z = 82)?
A) 1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p2
B) [Xe]6s26d106p2
C) [Xe]6s26f146d106p2
D) [Xe]6s24f145d106p2
E) [Xe]6s26p2
A) 1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p2
B) [Xe]6s26d106p2
C) [Xe]6s26f146d106p2
D) [Xe]6s24f145d106p2
E) [Xe]6s26p2

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
77
Which orbital diagram for the ground state electronic configuration of a carbon atom is correct?
(Remember Hund's Rule)
A)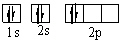
B)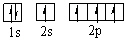
C)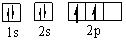
D)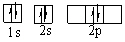
E)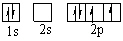
(Remember Hund's Rule)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
78
The element with a ground state electron configuration of 1s22s22p1 is
A) B
B) C
C) O
D) F
E) Ne
A) B
B) C
C) O
D) F
E) Ne

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
79
How many electrons can occupy a single 3px atomic orbital ?
A) zero
B) one
C) two
D) three
E) six
A) zero
B) one
C) two
D) three
E) six

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
80
The element with a ground state electron configuration of 1s22s22p4 is
A) B
B) C
C) O
D) F
E) Ne
A) B
B) C
C) O
D) F
E) Ne

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck


