Deck 8: The Periodic Table: Structure and Trends
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/90
Play
Full screen (f)
Deck 8: The Periodic Table: Structure and Trends
1
What is the highest energy subshell that is occupied in the ground state of an atom of Bi (at. no. = 83)?
A) 5p
B) 5d
C) 5f
D) 6p
E) none of these
A) 5p
B) 5d
C) 5f
D) 6p
E) none of these
6p
2
Exhibit 8-1 The following question(s) relate to the diagram below:
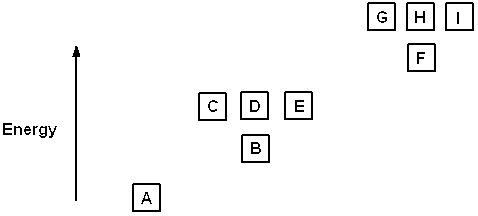
Refer to Exhibit 8-1. The three quantum numbers that describe the state labeled F are:
A) n = 2,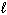 = 0,
= 0, 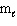 = 0
= 0
B) n = 3,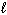 = 1,
= 1, 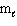 = 1
= 1
C) n = 2,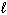 = 1,
= 1, 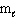 = 1
= 1
D) n = 3,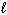 = 0,
= 0, 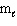 = 0
= 0
E) none of these

Refer to Exhibit 8-1. The three quantum numbers that describe the state labeled F are:
A) n = 2,
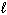 = 0,
= 0, 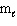 = 0
= 0B) n = 3,
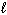 = 1,
= 1, 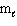 = 1
= 1C) n = 2,
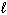 = 1,
= 1, 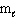 = 1
= 1D) n = 3,
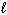 = 0,
= 0, 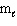 = 0
= 0E) none of these
n = 3, 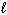 = 0,
= 0, 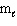 = 0
= 0
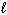 = 0,
= 0, 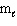 = 0
= 0 3
The ground state electron configuration of Ti is
A) 1s22s22p63s23p63d4
B) 1s22s22p63s23p64s13d3
C) 1s22s22p63s23p64s23d2
D) 1s22s22p63s23p64p4
E) none of these
A) 1s22s22p63s23p63d4
B) 1s22s22p63s23p64s13d3
C) 1s22s22p63s23p64s23d2
D) 1s22s22p63s23p64p4
E) none of these
1s22s22p63s23p64s23d2
4
Exhibit 8-1 The following question(s) relate to the diagram below:
Refer to Exhibit 8-1. Given the three statements below, pick the best answer.
I. The highest energy electron for Al would be in box F.
II. The highest energy electron for Be would be in box B.
III. The highest energy electron for He would be in box B.
A) II and III are true, I is false
B) I and II are true, III is false
C) only II is true
D) all three are true
E) all three are false

Refer to Exhibit 8-1. Given the three statements below, pick the best answer.
I. The highest energy electron for Al would be in box F.
II. The highest energy electron for Be would be in box B.
III. The highest energy electron for He would be in box B.
A) II and III are true, I is false
B) I and II are true, III is false
C) only II is true
D) all three are true
E) all three are false

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
5
Given the three statements below, pick the best answer.
I. For the H atom, the energy states are dependent only on n.
II. Knowing the values of n,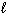 ,
, 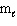 specifies an orbital.
specifies an orbital.
III. The highest energy electron for a K atom is located in a 4s orbital.
A) I and II are true, III is not
B) II and III are true, I is not
C) I and III are true, II is not
D) all three are true
E) only II is true
I. For the H atom, the energy states are dependent only on n.
II. Knowing the values of n,
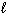 ,
, 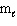 specifies an orbital.
specifies an orbital.III. The highest energy electron for a K atom is located in a 4s orbital.
A) I and II are true, III is not
B) II and III are true, I is not
C) I and III are true, II is not
D) all three are true
E) only II is true

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
6
Exhibit 8-1 The following question(s) relate to the diagram below:
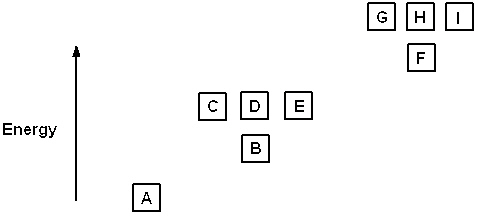
Refer to Exhibit 8-1. The three quantum numbers that describe the orbital labeled B are:
A) n = 1,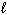 = 1,
= 1, 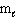 = 1
= 1
B) n = 2,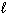 = 2,
= 2, 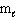 = 2
= 2
C) n = 2,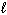 = 1,
= 1, 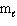 = - 1
= - 1
D) n = 2,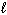 = 0,
= 0, 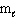 = 0
= 0
E) none of these

Refer to Exhibit 8-1. The three quantum numbers that describe the orbital labeled B are:
A) n = 1,
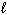 = 1,
= 1, 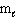 = 1
= 1B) n = 2,
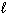 = 2,
= 2, 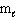 = 2
= 2C) n = 2,
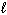 = 1,
= 1, 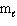 = - 1
= - 1D) n = 2,
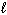 = 0,
= 0, 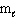 = 0
= 0E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
7
Exhibit 8-1 The following question(s) relate to the diagram below:
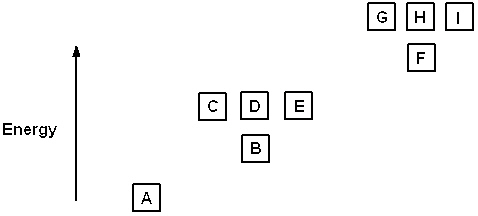
Refer to Exhibit 8-1. If boxes A, B, C, and D each are filled with two electrons and box E has one, the neutral atom that would have this arrangement of electrons is:
A) Ne
B) N
C) O
D) F
E) none of these

Refer to Exhibit 8-1. If boxes A, B, C, and D each are filled with two electrons and box E has one, the neutral atom that would have this arrangement of electrons is:
A) Ne
B) N
C) O
D) F
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
8
Exhibit 8-1 The following question(s) relate to the diagram below:
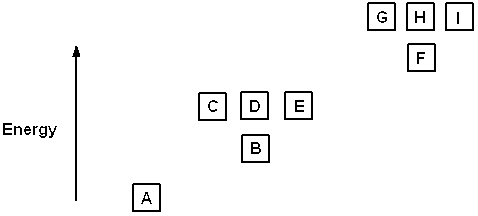
Refer to Exhibit 8-1. If all the boxes shown contain as many electrons as possible, what neutral element has that energy level diagram?
A) Ca
B) Ar
C) Mg
D) K
E) none of these

Refer to Exhibit 8-1. If all the boxes shown contain as many electrons as possible, what neutral element has that energy level diagram?
A) Ca
B) Ar
C) Mg
D) K
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
9
The ground state electron configuration 1s22s22p63s23p2 would be correct for which species listed below?
A) Al
B) P
C) P+
D) S
E) none of these
A) Al
B) P
C) P+
D) S
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
10
The ground state electron configuration of P+ is:
A) 1s22s22p43s23p4
B) [Ne]3s23p2
C) [Ne]3s23p4
D) [Ne]2s22p3
E) none of these
A) 1s22s22p43s23p4
B) [Ne]3s23p2
C) [Ne]3s23p4
D) [Ne]2s22p3
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
11
Exhibit 8-1 The following question(s) relate to the diagram below:
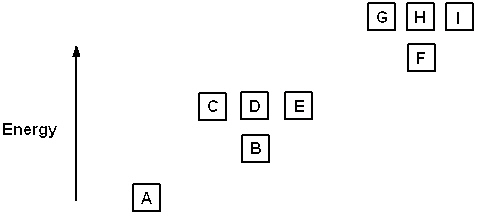
Refer to Exhibit 8-1. What set of quantum numbers are acceptable for box C?
A) n = 2,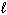 = 2,
= 2, 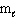 = - 2
= - 2
B) n = 3,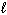 = 2,
= 2, 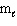 = - 2
= - 2
C) n = 1,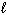 = 1,
= 1, 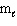 = - 1
= - 1
D) n = 3,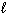 = 0,
= 0, 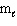 = - 1
= - 1
E) none are acceptable

Refer to Exhibit 8-1. What set of quantum numbers are acceptable for box C?
A) n = 2,
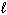 = 2,
= 2, 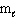 = - 2
= - 2B) n = 3,
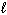 = 2,
= 2, 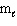 = - 2
= - 2C) n = 1,
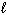 = 1,
= 1, 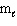 = - 1
= - 1D) n = 3,
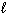 = 0,
= 0, 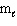 = - 1
= - 1E) none are acceptable

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
12
The ground state electron configuration of the oxide ion, O2 - , may be represented as:
A) 1s22s22p6
B) 1s22s22p43s2
C) 1s22s22p4
D) 1s22s22p23s23p2
E) none of these
A) 1s22s22p6
B) 1s22s22p43s2
C) 1s22s22p4
D) 1s22s22p23s23p2
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
13
The ground state of which species below has the most unpaired electrons?
A) P
B) O
C) Se
D) Fe
E) N
A) P
B) O
C) Se
D) Fe
E) N

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
14
The ground state electron configuration of Co is:
A) [Ar]4s23d4
B) [Ar]3d5
C) [Ar]3d6
D) [Ar]4s23d7
E) none of these
A) [Ar]4s23d4
B) [Ar]3d5
C) [Ar]3d6
D) [Ar]4s23d7
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
15
How many electrons would be in the n = 3 level for a Si atom?
A) 1
B) 2
C) 3
D) 4
E) none of these
A) 1
B) 2
C) 3
D) 4
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
16
The ground state electron configuration of Se is:
A) [Ar]3s23d103p4
B) [Ar]4s24d104p4
C) [Ar]4s23d104p4
D) [Ar]4s23d104p5
E) none of these
A) [Ar]3s23d103p4
B) [Ar]4s24d104p4
C) [Ar]4s23d104p4
D) [Ar]4s23d104p5
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is an acceptable orbital diagram for the valence shell of a carbon (Z = 6) atom in its ground state?
A)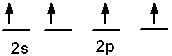
B)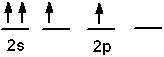
C)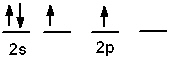
D)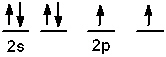
E) none of these
A)
B)
C)
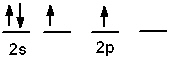
D)
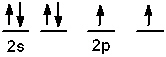
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
18
The valence shell orbital configuration of the Si atom in its ground state is:
A)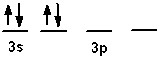
B)
C)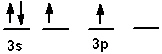
D)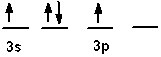
E) none of these
A)
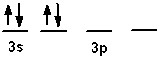
B)
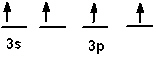
C)
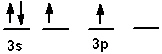
D)
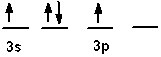
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
19
The highest energy electron for Ga (Z = 31) could be designated by which of the following four quantum numbers (only one correct possibility is shown, others are possible)?
A) n = 3,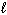 = 1,
= 1, 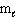 = 1, m s = 1/2
= 1, m s = 1/2
B) n = 4,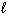 = 1,
= 1, 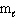 = - 1, m s = 1/2
= - 1, m s = 1/2
C) n = 3,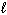 = 0,
= 0, 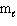 = 0, m s = 1/2
= 0, m s = 1/2
D) n = 4,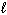 = 2,
= 2, 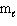 = 1, m s = 1/2
= 1, m s = 1/2
E) n = 5,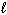 = 2,
= 2, 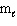 = 1, m s = 1/2
= 1, m s = 1/2
A) n = 3,
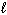 = 1,
= 1, 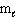 = 1, m s = 1/2
= 1, m s = 1/2B) n = 4,
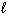 = 1,
= 1, 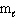 = - 1, m s = 1/2
= - 1, m s = 1/2C) n = 3,
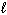 = 0,
= 0, 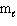 = 0, m s = 1/2
= 0, m s = 1/2D) n = 4,
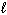 = 2,
= 2, 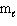 = 1, m s = 1/2
= 1, m s = 1/2E) n = 5,
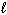 = 2,
= 2, 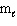 = 1, m s = 1/2
= 1, m s = 1/2
Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
20
The ground state electron configuration of Co3+ is:
A) 1s22s22p63s23p64s23d4
B) 1s22s22p63s23p63d5
C) 1s22s22p63s23p63d4
D) 1s22s22p63s23p63d6
E) none of these
A) 1s22s22p63s23p64s23d4
B) 1s22s22p63s23p63d5
C) 1s22s22p63s23p63d4
D) 1s22s22p63s23p63d6
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
21
How many unpaired electrons does the ground state of the species S+ have?
A) 1
B) 2
C) 3
D) 0
E) none of these
A) 1
B) 2
C) 3
D) 0
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
22
The correct ground state electron configuration for Ni2+ is:
A) [Ar]4s23d8
B) [Ar]3d7
C) [Ar]3d8
D) [Ar]4s23d6
E) none of these
A) [Ar]4s23d8
B) [Ar]3d7
C) [Ar]3d8
D) [Ar]4s23d6
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
23
How many valence electrons does a silicon atom possess?
A) 2
B) 4
C) 6
D) 14
E) 16
A) 2
B) 4
C) 6
D) 14
E) 16

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
24
How many unpaired electrons does the ground state of the species Al+ have?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
25
What ion has the ground state electron configuration [Ar]4s23d3?
A) Fe2+
B) Co3+
C) Cr+
D) Mn2+
E) none of these
A) Fe2+
B) Co3+
C) Cr+
D) Mn2+
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
26
How many valence electrons are present in an atom of Arsenic ?
A) 2
B) 3
C) 5
D) 13
E) 33
A) 2
B) 3
C) 5
D) 13
E) 33

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
27
How many unpaired electrons does the ground state of S have?
A) 1
B) 2
C) 3
D) 4
E) 0
A) 1
B) 2
C) 3
D) 4
E) 0

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
28
Which species listed below is not isoelectronic with Br1 - ?
A) Kr
B) Cl1 -
C) Se2 -
D) Rb1+
E) Sr2+
A) Kr
B) Cl1 -
C) Se2 -
D) Rb1+
E) Sr2+

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
29
How many valence electrons does the ground state of P have?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
30
Which element has the valence electrons 3s23p2?
A) Al
B) Si
C) P
D) Ga
E) none of these
A) Al
B) Si
C) P
D) Ga
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
31
The ground state electron configuration [Ar]3d4 is correct for:
A) Mn2+
B) Fe3+
C) Cr3+
D) Mn3+
E) none of these
A) Mn2+
B) Fe3+
C) Cr3+
D) Mn3+
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is not isoelectronic with the rest?
A) Sc3+
B) Ca
C) K+
D) Ar
E) all are isoelectronic
A) Sc3+
B) Ca
C) K+
D) Ar
E) all are isoelectronic

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
33
What is the ground state electron configuration of Cr2+?
A) [Ar]4s13d3
B) [Ar]4s23d2
C) [Ar]3d4
D) [Ar]3d3
E) none of these
A) [Ar]4s13d3
B) [Ar]4s23d2
C) [Ar]3d4
D) [Ar]3d3
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
34
The ground state electron configuration of Ca is:
A) 1s22s22p63s23p63d2
B) [Ar]3s2
C) [Ar]3d2
D) 1s22s22p63s23p64s2
E) none of these
A) 1s22s22p63s23p63d2
B) [Ar]3s2
C) [Ar]3d2
D) 1s22s22p63s23p64s2
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
35
Of the three species, which would be isoelectronic with O?
I. N+
II. F -
III. Ne2 -
A) I and II are isoelectronic, III is not isoelectronic with O
B) only I is isoelectronic with O
C) only II is isoelectronic with O
D) all are isoelectronic with O
E) none are isoelectronic with O
I. N+
II. F -
III. Ne2 -
A) I and II are isoelectronic, III is not isoelectronic with O
B) only I is isoelectronic with O
C) only II is isoelectronic with O
D) all are isoelectronic with O
E) none are isoelectronic with O

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
36
What are the valence electrons for Ge?
A) 4s23d104p2
B) 3s23p2
C) 4s23d10
D) 4s24p2
E) none of these
A) 4s23d104p2
B) 3s23p2
C) 4s23d10
D) 4s24p2
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
37
The ground state electron configuration of the ion, P+, may be represented as:
A) [Ne]3s23p2
B) [Ne]3s23p4
C) 1s22s22p63s23p3
D) [Ne]3s23p6
E) none of these
A) [Ne]3s23p2
B) [Ne]3s23p4
C) 1s22s22p63s23p3
D) [Ne]3s23p6
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
38
How many unpaired electrons does the ground state of Fe3+ have?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
39
What is the value of n (principal quantum number) for a valence electron in a ground state Ca atom?
A) 1
B) 2
C) 3
D) 4
E) 0
A) 1
B) 2
C) 3
D) 4
E) 0

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
40
Which element has the valence electrons 4s23d2?
A) Ca
B) Sc
C) Ti
D) V
E) none of these
A) Ca
B) Sc
C) Ti
D) V
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
41
Which species is isoelectronic with Al?
A) Si2+
B) Si -
C) P2 -
D) P2+
E) none of these
A) Si2+
B) Si -
C) P2 -
D) P2+
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
42
Three series of atoms are listed below in order of increasing size. Pick the best answer.
I. Li
II. B
III. Cl
A) I and II are correct, III is not
B) I and III are correct, II is not
C) II and III are correct, I is not
D) all three are correct
E) only III is correct
I. Li
II. B
III. Cl
A) I and II are correct, III is not
B) I and III are correct, II is not
C) II and III are correct, I is not
D) all three are correct
E) only III is correct

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
43
Arrange the elements Al, Na, and P in order of increasing atomic radii based upon their position on the periodic table.
A) Al < Na < P
B) Na < Al < P
C) P < Al < Na
D) P < Na < Al
E) Na < P < Al
A) Al < Na < P
B) Na < Al < P
C) P < Al < Na
D) P < Na < Al
E) Na < P < Al

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
44
Arrange the species Cl - 1, K+1, and S - 2 in terms of increasing radii .
A) Cl - 1 - 2
B) K+1 - 1 - 2
C) Cl - 1 - 2 < K+1
D) K+1 - 2- 1
E) S - 2 - 1< K+1
A) Cl - 1 - 2
B) K+1 - 1 - 2
C) Cl - 1 - 2 < K+1
D) K+1 - 2- 1
E) S - 2 - 1< K+1

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
45
Which one of the following has the largest atomic radius ?
A) S2 -
B) Ca2+
C) Cl1 -
D) Ar
E) K1+
A) S2 -
B) Ca2+
C) Cl1 -
D) Ar
E) K1+

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
46
Which species below is largest?
A) Cl
B) S2 -
C) K+
D) S
E) Cl -
A) Cl
B) S2 -
C) K+
D) S
E) Cl -

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
47
Which answer lists the atoms shown in order of increasing size?
A) P < S < Cl
B) S < Se < Br
C) O < P < Ge
D) P < Cl < Se
E) none of these
A) P < S < Cl
B) S < Se < Br
C) O < P < Ge
D) P < Cl < Se
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following series of atoms are arranged in order of increasing size?
A) H < Li < Be
B) Na < Mg < Ca
C) Cl < Br < As
D) K < Ga < In
E) none of these
A) H < Li < Be
B) Na < Mg < Ca
C) Cl < Br < As
D) K < Ga < In
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
49
Which species from the list below is isoelectronic with Na1+?
A) K1+
B) Ar
C) S2 -
D) N3 -
E) Ca2+
A) K1+
B) Ar
C) S2 -
D) N3 -
E) Ca2+

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
50
Arrange the following elements in order of increasing atomic radii . Barium, Calcium, Bromine
A) (smallest) Barium < Calcium < Bromine (largest)
B) (smallest) Barium < Bromine < Calcium (largest)
C) (smallest) Bromine < Calcium < Barium (largest)
D) (smallest) Calcium < Barium < Bromine (largest)
E) (smallest) Calcium < Bromine < Barium (largest)
A) (smallest) Barium < Calcium < Bromine (largest)
B) (smallest) Barium < Bromine < Calcium (largest)
C) (smallest) Bromine < Calcium < Barium (largest)
D) (smallest) Calcium < Barium < Bromine (largest)
E) (smallest) Calcium < Bromine < Barium (largest)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
51
Given the three statements below, pick the best answer.
I. Be is larger than Li
II. Be+ is larger than Li
III. O2 - is larger than F -
A) I and II are true, III is false
B) only III is true
C) I and III are true, II is false
D) II and III are true, I is false
E) all three are false
I. Be is larger than Li
II. Be+ is larger than Li
III. O2 - is larger than F -
A) I and II are true, III is false
B) only III is true
C) I and III are true, II is false
D) II and III are true, I is false
E) all three are false

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
52
What is the order for increasing atomic radius among the following three elements?
Tl, Al, Si
A) Tl < Al < Si
B) Tl < Si < Al
C) Si < Al < Tl
D) Si < Tl < Al
E) Al < Tl < Si
Tl, Al, Si
A) Tl < Al < Si
B) Tl < Si < Al
C) Si < Al < Tl
D) Si < Tl < Al
E) Al < Tl < Si

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
53
Which species shown below is the largest?
A) S
B) S2 -
C) Cl
D) Cl -
E) P3 -
A) S
B) S2 -
C) Cl
D) Cl -
E) P3 -

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
54
Arrange the ions Ca2+, Cl1 - , K1+ and S2 - in order of increasing size .
A) Ca2+ - -
B) Ca2+ - -
C) S2 - - < Ca2+
D) S2 - - < K1+ < Ca2+
E) K1+ - -
A) Ca2+ - -
B) Ca2+ - -
C) S2 - - < Ca2+
D) S2 - - < K1+ < Ca2+
E) K1+ - -

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
55
Which species is isoelectronic with As?
A) Ga2+
B) Se -
C) P
D) Ge -
E) none of these
A) Ga2+
B) Se -
C) P
D) Ge -
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
56
Which species is not isoelectronic with the remaining atoms or ions listed?
A) Kr
B) Rb+1
C) Na+1
D) Br - 1
E) Se - 2
A) Kr
B) Rb+1
C) Na+1
D) Br - 1
E) Se - 2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
57
Which species shown below is the largest?
A) P
B) S
C) Cl
D) N
E) O
A) P
B) S
C) Cl
D) N
E) O

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
58
Arrange the following species in order of increasing radii . N3 - , F - , Ne, Na+1, Mg2+
A) (smallest) N3 - < Ne < Na+1 < Mg2+ (largest)
B) (smallest) Mg2+ (largest)
C) (smallest) Ne (largest)
D) (smallest) F - < Mg2+ < Ne (largest)
E) (smallest) Na+1 < Ne < Mg2+ (largest)
A) (smallest) N3 - < Ne < Na+1 < Mg2+ (largest)
B) (smallest) Mg2+ (largest)
C) (smallest) Ne (largest)
D) (smallest) F - < Mg2+ < Ne (largest)
E) (smallest) Na+1 < Ne < Mg2+ (largest)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following species is not isoelectronic with Na+?
A) Mg2+
B) Li+
C) Ne
D) F1 -
E) O2 -
A) Mg2+
B) Li+
C) Ne
D) F1 -
E) O2 -

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following series of atoms are arranged in order of decreasing size?
A) K > Ga > In
B) Cl > Br > Kr
C) Li > Mg > Ca
D) H > He > Li
E) none of these
A) K > Ga > In
B) Cl > Br > Kr
C) Li > Mg > Ca
D) H > He > Li
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
61
Arrange the elements Ba, Be, and Ca in order of increasing first ionization energy based upon their position on the periodic table.
A) Ba < Be < Ca
B) Ba < Ca < Be
C) Be < Ca < Ba
D) Be < Ba < Ca
E) Ca < Ba < Be
A) Ba < Be < Ca
B) Ba < Ca < Be
C) Be < Ca < Ba
D) Be < Ba < Ca
E) Ca < Ba < Be

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
62
Which species shown below is largest?
A) Xe
B) Ba
C) Cs
D) Ba+
E) Rb
A) Xe
B) Ba
C) Cs
D) Ba+
E) Rb

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
63
Which element listed below would most likely have the following series of ionization energies?
IE1 IE2 IE3 IE4 IE5 IE6 0.80 MJ/mol 2.43 MJ/mol 3.66 MJ/mol 6.22 MJ/mol 37.83 MJ/mol 47.28 MJ/mol
A) Li
B) B
C) C
D) O
E) F
IE1 IE2 IE3 IE4 IE5 IE6 0.80 MJ/mol 2.43 MJ/mol 3.66 MJ/mol 6.22 MJ/mol 37.83 MJ/mol 47.28 MJ/mol
A) Li
B) B
C) C
D) O
E) F

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
64
Arrange the following species in order of increasing radii . Cl+, Cl, Cl -
A) (smallest) Cl+ - (largest)
B) (smallest) Cl+ - < Cl (largest)
C) (smallest) Cl - (largest)
D) (smallest) Cl - < Cl+ (largest)
E) (smallest) Cl - < Cl < Cl+ (largest)
A) (smallest) Cl+ - (largest)
B) (smallest) Cl+ - < Cl (largest)
C) (smallest) Cl - (largest)
D) (smallest) Cl - < Cl+ (largest)
E) (smallest) Cl - < Cl < Cl+ (largest)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
65
Arrange the following elements in order of increasing ionization energy. O, Mg, He
A) (lowest IE) O < Mg < He (highest IE)
B) (lowest IE) O < He < Mg (highest IE)
C) (lowest IE) He < O < Mg (highest IE)
D) (lowest IE) He < Mg < O (highest IE)
E) (lowest IE) Mg < O < He (highest IE)
A) (lowest IE) O < Mg < He (highest IE)
B) (lowest IE) O < He < Mg (highest IE)
C) (lowest IE) He < O < Mg (highest IE)
D) (lowest IE) He < Mg < O (highest IE)
E) (lowest IE) Mg < O < He (highest IE)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
66
Given the three statements below, pick the best answer.
I. The Zeff for the highest energy electron in Be is higher than the Zeff for the highest energy electron for N.
II. The second ionization energy for Mg is greater than the first ionization energy of Na.
III. The species O2 - , Ne, and Na are isoelectronic.
A) only I is true
B) only II is true
C) II and III are true, I is false
D) I and III are true, II is false
E) I and II are true, III is false
I. The Zeff for the highest energy electron in Be is higher than the Zeff for the highest energy electron for N.
II. The second ionization energy for Mg is greater than the first ionization energy of Na.
III. The species O2 - , Ne, and Na are isoelectronic.
A) only I is true
B) only II is true
C) II and III are true, I is false
D) I and III are true, II is false
E) I and II are true, III is false

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
67
The answer which correctly arranges the species N, N - , O+, F2+ in order of increasing ionization energy is:
A) N - < F2+
B) O+ - < F2+
C) N - < N < O+ < F2+
D) F2+ -
E) none of these is correct
A) N - < F2+
B) O+ - < F2+
C) N - < N < O+ < F2+
D) F2+ -
E) none of these is correct

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is true?
I. A neutral atom is larger than its corresponding cation. (for example:
Na > Na+)
II. A neutral atom is larger than its corresponding anion. (for example:
O > O2 - )
III. Ionic radii increase down a group. (for example:
Li+
A) I only
B) I and II
C) I and III
D) II and III
E) All of these
I. A neutral atom is larger than its corresponding cation. (for example:
Na > Na+)
II. A neutral atom is larger than its corresponding anion. (for example:
O > O2 - )
III. Ionic radii increase down a group. (for example:
Li+
A) I only
B) I and II
C) I and III
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following processes listed below represents the experiment for measuring an ionization energy ?
("E" represents a neutral E lement, "M" represents a M etal cation and "X" represents a nonmetal anion)
A) E (g) + e - → E - (g) + energy
B) E (g) + energy→ E+ (g) + e -
C) MX (g) + energy→ Mn+ (g) + Xn - (g)
D) X - X (g) + energy→ 2 X · (g)
E) None of these
("E" represents a neutral E lement, "M" represents a M etal cation and "X" represents a nonmetal anion)
A) E (g) + e - → E - (g) + energy
B) E (g) + energy→ E+ (g) + e -
C) MX (g) + energy→ Mn+ (g) + Xn - (g)
D) X - X (g) + energy→ 2 X · (g)
E) None of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
70
Arrange the elements Ne, P and K in order of increasing first ionization energy .
A) Ne < P < K
B) Ne < K < P
C) K < P < Ne
D) K < Ne < P
E) P < Ne < K
A) Ne < P < K
B) Ne < K < P
C) K < P < Ne
D) K < Ne < P
E) P < Ne < K

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
71
Which answer below correctly lists the atoms shown in order of increasing first ionization energy?
A) B < N < Be
B) C < Be < Li
C) B < N < F
D) C < Be < N
E) none of these
A) B < N < Be
B) C < Be < Li
C) B < N < F
D) C < Be < N
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the series written below is in order of increasing first ionization energy (atoms with the smallest ionization energy first)?
A) K < P < Ca
B) F < N < B
C) Ca < S < F
D) Be < F < P
E) none of these
A) K < P < Ca
B) F < N < B
C) Ca < S < F
D) Be < F < P
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
73
Which series of species shown below is arranged in order of increasing ionization energy?
A) Li < Si < Cl < Ar+
B) K < Al < Ga < Ca
C) Si < Ge < Ga < K
D) O < C < Be < Li
E) none are in order of increasing ionization energy
A) Li < Si < Cl < Ar+
B) K < Al < Ga < Ca
C) Si < Ge < Ga < K
D) O < C < Be < Li
E) none are in order of increasing ionization energy

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
74
Listed below are three rankings of atoms or ions in order of increasing ionization energies. Pick the best answer.
I. Li
II. P
III. Al
A) I and II are correct orders, III is not
B) I and III are correct orders, II is not
C) II and III are correct orders, I is not
D) all three are correct
E) only I is correct
I. Li
II. P
III. Al
A) I and II are correct orders, III is not
B) I and III are correct orders, II is not
C) II and III are correct orders, I is not
D) all three are correct
E) only I is correct

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
75
Arrange the following elements in order of increasing first ionization energy . Br, Cl, Se
A) (lowest IE) Br < Cl < Se (highest IE)
B) (lowest IE) Br < Se < Cl (highest IE)
C) (lowest IE) Se < Cl < Br (highest IE)
D) (lowest IE) Se < Br < Cl (highest IE)
E) (lowest IE) Cl < Br < Se (highest IE)
A) (lowest IE) Br < Cl < Se (highest IE)
B) (lowest IE) Br < Se < Cl (highest IE)
C) (lowest IE) Se < Cl < Br (highest IE)
D) (lowest IE) Se < Br < Cl (highest IE)
E) (lowest IE) Cl < Br < Se (highest IE)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
76
Listed below are three rankings of atoms or ions in order of increasing ionization energy. Pick the correct answer.
I. B
II. C
III. Al
A) I and III are true, II is false
B) I and II are true, III is false
C) II and III are true, I is false
D) only II is true
E) only III is true
I. B
II. C
III. Al
A) I and III are true, II is false
B) I and II are true, III is false
C) II and III are true, I is false
D) only II is true
E) only III is true

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
77
Which series shown below is correctly arranged in order of increasing ionization energy?
A) Na < K < Ga < Se
B) Ca < Mg < Al < S
C) Li < B < N < Be
D) I < Te < Y < Rb < I
E) none of these
A) Na < K < Ga < Se
B) Ca < Mg < Al < S
C) Li < B < N < Be
D) I < Te < Y < Rb < I
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
78
Which species shown below is largest?
A) In
B) Sb
C) In+
D) Sn+
E) Sn
A) In
B) Sb
C) In+
D) Sn+
E) Sn

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
79
Which series shown below is correctly arranged in order of increasing ionization energy?
A) Na < Al < Cl < Si
B) Cl < Si < Al < Na
C) Li < B < N < F
D) In < Te < Rb < I
E) none of these
A) Na < Al < Cl < Si
B) Cl < Si < Al < Na
C) Li < B < N < F
D) In < Te < Rb < I
E) none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
80
Element "Q" is a third period element and has the following successive ionization energies. What is element "Q"?
IE1 IE2 IE3 IE4 IE5 IE6 577 kJ/mol 1816 kJ/mol 2744 kJ 11,576 kJ/mol 14,829 kJ/mol 18,375 kJ/mol
A) Na
B) Mg
C) Al
D) Ga
E) Si
IE1 IE2 IE3 IE4 IE5 IE6 577 kJ/mol 1816 kJ/mol 2744 kJ 11,576 kJ/mol 14,829 kJ/mol 18,375 kJ/mol
A) Na
B) Mg
C) Al
D) Ga
E) Si

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck


