Deck 5: Thermochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/78
Play
Full screen (f)
Deck 5: Thermochemistry
1
Given the thermochemical equation:
2 H₂ O₂ (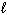 ) 2 H₂ O (
) 2 H₂ O ( 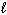 ) + O₂(g) D H = - 189 kJ What is D H for the formation of 8.00 g of O₂?
) + O₂(g) D H = - 189 kJ What is D H for the formation of 8.00 g of O₂?
A) - 22.2 kJ
B) - 47.2 kJ
C) - 94.5 kJ
D) - 189 kJ
E) none of these
2 H₂ O₂ (
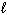 ) 2 H₂ O (
) 2 H₂ O ( 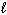 ) + O₂(g) D H = - 189 kJ What is D H for the formation of 8.00 g of O₂?
) + O₂(g) D H = - 189 kJ What is D H for the formation of 8.00 g of O₂?A) - 22.2 kJ
B) - 47.2 kJ
C) - 94.5 kJ
D) - 189 kJ
E) none of these
- 47.2 kJ
2
What mass of ethane gas (C₂ H₆ ) must burn in excess oxygen to produce 2.00 10 3 kJ of heat, given the thermochemical equation:
2 C₂ H₆(g) + 7 O₂(g) 4 CO₂ (g) + 6 H₂ O (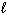 ) D H = - 3.12 10 3 kJ
) D H = - 3.12 10 3 kJ
A) 0.641 g
B) 19.3 g
C) 38.6 g
D) heat is not produced by this reaction
E) none of these
2 C₂ H₆(g) + 7 O₂(g) 4 CO₂ (g) + 6 H₂ O (
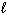 ) D H = - 3.12 10 3 kJ
) D H = - 3.12 10 3 kJA) 0.641 g
B) 19.3 g
C) 38.6 g
D) heat is not produced by this reaction
E) none of these
38.6 g
3
Given the equation below, what is D H for the reaction that converts 6.00 g of H₂ O ( 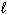 ) into H₂ O₂ (
) into H₂ O₂ ( 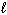 ) and H₂ (g)?
) and H₂ (g)?
2 H₂ O (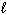 ) H₂ O₂ (
) H₂ O₂ ( 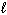 ) + H₂ (g) D H = 384 kJ
) + H₂ (g) D H = 384 kJ
A) 129 kJ
B) 64.0 kJ
C) 256 kJ
D) 384 kJ
E) none of these
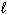 ) into H₂ O₂ (
) into H₂ O₂ ( 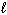 ) and H₂ (g)?
) and H₂ (g)?2 H₂ O (
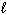 ) H₂ O₂ (
) H₂ O₂ ( 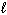 ) + H₂ (g) D H = 384 kJ
) + H₂ (g) D H = 384 kJA) 129 kJ
B) 64.0 kJ
C) 256 kJ
D) 384 kJ
E) none of these
64.0 kJ
4
Consider the following reaction and its corresponding enthalpy:
2 Mg (s) + O2 (g)→2 MgO (s) D H ° rxn = - 1204 kJ/mol What amount of heat is exchanged when 5.00 grams of magnesium metal reacts at constant pressure?
A) - 124 kJ
B) - 248 kJ
C) - 3.01×103 kJ
D) 7.32×104 kJ
E) 1.44×105 kJ
2 Mg (s) + O2 (g)→2 MgO (s) D H ° rxn = - 1204 kJ/mol What amount of heat is exchanged when 5.00 grams of magnesium metal reacts at constant pressure?
A) - 124 kJ
B) - 248 kJ
C) - 3.01×103 kJ
D) 7.32×104 kJ
E) 1.44×105 kJ

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
5
Given the equation below, what is D H for burning 10.0 g of CO (g) in excess oxygen?
2 CO (g) + O2 (g)→2 CO2 (g) D H = - 566 kJ
A) 101 kJ
B) 202 kJ
C) 50.5 kJ
D) 142 kJ
E) none of these
2 CO (g) + O2 (g)→2 CO2 (g) D H = - 566 kJ
A) 101 kJ
B) 202 kJ
C) 50.5 kJ
D) 142 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
6
What mass of propane gas (C3 H8 ) must burn in excess oxygen to produce 9.00 10 3 kJ of heat, given the thermochemical equation:
C3 H8 (g) + 5 O₂(g) 3 CO₂ (g) + 4 H₂ O (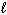 ) D H = - 2.22 10 3 kJ
) D H = - 2.22 10 3 kJ
A) 1.79×10 g
B) 35.8 g
C) 4.05 g
D) heat is not produced by this reaction
E) none of these
C3 H8 (g) + 5 O₂(g) 3 CO₂ (g) + 4 H₂ O (
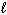 ) D H = - 2.22 10 3 kJ
) D H = - 2.22 10 3 kJA) 1.79×10 g
B) 35.8 g
C) 4.05 g
D) heat is not produced by this reaction
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
7
What mass of nitrogen must burn in excess oxygen to produce 18.0 kJ of heat, given the following thermochemical equation?
N2 (g) + O2 (g)→2 NO (g) D H = +1.80×102 kJ
A) 0.100 g
B) 2.80 g
C) 5.60 g
D) heat is not released by this reaction
E) none of these
N2 (g) + O2 (g)→2 NO (g) D H = +1.80×102 kJ
A) 0.100 g
B) 2.80 g
C) 5.60 g
D) heat is not released by this reaction
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
8
Liquid hydrogen peroxide is an oxidizing agent in many rocket fuel mixtures because it releases oxygen gas on decomposition as shown below. 2 H₂ O₂ ( 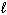 ) 2 H₂ O (
) 2 H₂ O ( 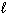 ) + O₂(g) D H rxn = - 196 kJ How much heat is released when 25.0 grams of H₂ O₂ decomposes?
) + O₂(g) D H rxn = - 196 kJ How much heat is released when 25.0 grams of H₂ O₂ decomposes?
Molar Mass(H₂ O₂) = 34.01 g/mol
A) - 533 kJ
B) - 288 kJ
C) - 144 kJ
D) - 133 kJ
E) - 72.0 kJ
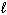 ) 2 H₂ O (
) 2 H₂ O ( 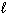 ) + O₂(g) D H rxn = - 196 kJ How much heat is released when 25.0 grams of H₂ O₂ decomposes?
) + O₂(g) D H rxn = - 196 kJ How much heat is released when 25.0 grams of H₂ O₂ decomposes?Molar Mass(H₂ O₂) = 34.01 g/mol
A) - 533 kJ
B) - 288 kJ
C) - 144 kJ
D) - 133 kJ
E) - 72.0 kJ

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
9
Given the following reaction and its corresponding enthalpy of reaction, how many grams of Fe2O3 are produced when 5.62×103 kJ is released to the surroundings?
Molar Mass (Fe2O3) = 159.7 g/mol 4 Fe (s) + 3 O2 (g)→2 Fe2O3 (s) D H rxn = - 1.65×103 kJ
A) 46.9 g Fe2O3
B) 93.8 g Fe2O3
C) 272 g Fe2O3
D) 544 g Fe2O3
E) 1.09×103 g Fe2O3
Molar Mass (Fe2O3) = 159.7 g/mol 4 Fe (s) + 3 O2 (g)→2 Fe2O3 (s) D H rxn = - 1.65×103 kJ
A) 46.9 g Fe2O3
B) 93.8 g Fe2O3
C) 272 g Fe2O3
D) 544 g Fe2O3
E) 1.09×103 g Fe2O3

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
10
Energy possessed by a sample of matter because of its position or condition is called
A) chemical energy
B) potential energy
C) kinetic energy
D) heat
E) none of these
A) chemical energy
B) potential energy
C) kinetic energy
D) heat
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following reactions releases heat to the surroundings as it proceeds to completion?
I. C (coal) + H2O (g) + 130 KJ→CO (g) + H2 (g)
II. 2 NO2 (g)→N2O4 (exothermic)
III. 2 H2 (g) + O2 (g)→2 H2O (g) D H rxn = - 572 kJ
A) I only
B) I and III
C) I and II
D) II and III
E) All of these
I. C (coal) + H2O (g) + 130 KJ→CO (g) + H2 (g)
II. 2 NO2 (g)→N2O4 (exothermic)
III. 2 H2 (g) + O2 (g)→2 H2O (g) D H rxn = - 572 kJ
A) I only
B) I and III
C) I and II
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
12
Given the three statements below, which answer is correct?
I. A thermochemical equation must include the physical state of all substances.
II. The coefficients in a thermochemical equation are interpreted as moles.
III. The sign of D H is negative for an endothermic reaction.
A) I and II are true, III is false
B) I and III are true, II is false
C) I, II, and III are all true
D) I, II, and III are all false
E) II and III are true, I is false
I. A thermochemical equation must include the physical state of all substances.
II. The coefficients in a thermochemical equation are interpreted as moles.
III. The sign of D H is negative for an endothermic reaction.
A) I and II are true, III is false
B) I and III are true, II is false
C) I, II, and III are all true
D) I, II, and III are all false
E) II and III are true, I is false

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
13
Given the equation below, what is D H for burning 14.0 g of CO (g) in excess oxygen?
2 CO (g) + O2 (g)→2 CO2 (g) D H = - 566 kJ
A) - 566 kJ
B) - 283 kJ
C) - 142 kJ
D) +283 kJ
E) none of these
2 CO (g) + O2 (g)→2 CO2 (g) D H = - 566 kJ
A) - 566 kJ
B) - 283 kJ
C) - 142 kJ
D) +283 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
14
Given the thermochemical equation:
2 SO2 (g) + O2 (g)→2 SO3 (g) D H = - 197 kJ What is D H for the formation of 8.00 g of SO3?
A) - 9.85 kJ
B) - 19.7 kJ
C) - 39.4 kJ
D) - 197 kJ
E) none of these
2 SO2 (g) + O2 (g)→2 SO3 (g) D H = - 197 kJ What is D H for the formation of 8.00 g of SO3?
A) - 9.85 kJ
B) - 19.7 kJ
C) - 39.4 kJ
D) - 197 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
15
Given the equation below, what is D H for burning 8.0 g of C₂ H₂ (g) in excess O₂(g)?
2 C₂ H₂ (g) + 5 O₂(g) 4 CO₂ (g) + 2 H₂ O (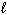 ) D H = - 2600 kJ
) D H = - 2600 kJ
A) - 200 kJ
B) - 400 kJ
C) - 800 kJ
D) - 1600 kJ
E) none of these
2 C₂ H₂ (g) + 5 O₂(g) 4 CO₂ (g) + 2 H₂ O (
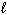 ) D H = - 2600 kJ
) D H = - 2600 kJA) - 200 kJ
B) - 400 kJ
C) - 800 kJ
D) - 1600 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
16
Exhibit 5-1 Consider the reaction of Magnesium with oxygen to form magnesium oxide and heat as shown below to answer the following problem(s):
2 Mg (s) + O2 (g)→2 MgO (s) D H ° rxn = - 1204 kJ
Refer to Exhibit 5-1. How many grams of MgO are produced during an enthalpy change of - 96.0 kJ?
Molar Mass(MgO) = 40.3 g/mol
A) 1010 g
B) 6.43 g
C) 3.21 g
D) 0.311 g
E) None of these
2 Mg (s) + O2 (g)→2 MgO (s) D H ° rxn = - 1204 kJ
Refer to Exhibit 5-1. How many grams of MgO are produced during an enthalpy change of - 96.0 kJ?
Molar Mass(MgO) = 40.3 g/mol
A) 1010 g
B) 6.43 g
C) 3.21 g
D) 0.311 g
E) None of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following reactions absorbs heat from the surroundings in order to proceed to completion?
I. CO (g) + 1/2 O2 (g)→CO2 (g) D H rxn = - 283.0 kJ
II. Al2O3 (s)→2 Al (s) + 3/2 O2 (g) (endothermic)
III. SO3 (g) + 99.2 kJ→S (s) + 3/2 O2 (g)
A) I only
B) I and III
C) I and II
D) II and III
E) All of these
I. CO (g) + 1/2 O2 (g)→CO2 (g) D H rxn = - 283.0 kJ
II. Al2O3 (s)→2 Al (s) + 3/2 O2 (g) (endothermic)
III. SO3 (g) + 99.2 kJ→S (s) + 3/2 O2 (g)
A) I only
B) I and III
C) I and II
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
18
Exhibit 5-1 Consider the reaction of Magnesium with oxygen to form magnesium oxide and heat as shown below to answer the following problem(s):
2 Mg (s) + O2 (g)→2 MgO (s) D H ° rxn = - 1204 kJ
Refer to Exhibit 5-1. How much heat is released when 2.43 grams of Mg (s) reacts at constant pressure?
Molar Mass(Mg) = 24.3 g/mol
A) - 602 kJ
B) - 240.8 kJ
C) - 120.4 kJ
D) - 60.2 kJ
E) None of these
2 Mg (s) + O2 (g)→2 MgO (s) D H ° rxn = - 1204 kJ
Refer to Exhibit 5-1. How much heat is released when 2.43 grams of Mg (s) reacts at constant pressure?
Molar Mass(Mg) = 24.3 g/mol
A) - 602 kJ
B) - 240.8 kJ
C) - 120.4 kJ
D) - 60.2 kJ
E) None of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
19
Given the equation below, what is D H for burning 2.00 g of CO (g) in excess oxygen?
2 CO (g) + O2 (g)→2 CO2 (g) D H = - 566 kJ
A) - 10.1 kJ
B) - 28.4 kJ
C) - 20.2 kJ
D) - 40.4 kJ
E) none of these
2 CO (g) + O2 (g)→2 CO2 (g) D H = - 566 kJ
A) - 10.1 kJ
B) - 28.4 kJ
C) - 20.2 kJ
D) - 40.4 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
20
Given the three statements below, which answer is correct?
I. An exothermic reaction releases heat to the surroundings.
II. The sign of D H for an exothermic reaction is positive.
III. For an endothermic reaction to be carried out at constant temperature, heat must be supplied from the surroundings.
A) I and II are true, III is false
B) I and III are true, II is false
C) I, II, and III are all true
D) I, II, and III are all false
E) II and III are true, I is false
I. An exothermic reaction releases heat to the surroundings.
II. The sign of D H for an exothermic reaction is positive.
III. For an endothermic reaction to be carried out at constant temperature, heat must be supplied from the surroundings.
A) I and II are true, III is false
B) I and III are true, II is false
C) I, II, and III are all true
D) I, II, and III are all false
E) II and III are true, I is false

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
21
How many joules of heat are necessary to raise the temperature of a 1.42 kg block of copper from 25.0 ° C to 88.5 ° C?
(Specific heat of copper = 0.385 J/g × ° C)
A) 234000 J
B) 34700 J
C) 234 J
D) 34.7 J
E) 0.0347 J
(Specific heat of copper = 0.385 J/g × ° C)
A) 234000 J
B) 34700 J
C) 234 J
D) 34.7 J
E) 0.0347 J

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
22
When a 40.0 g sample of a metal at 25.00 ° C is added tO65.0 g of water at 100.00 ° C, the final temperature of both the water and metal is 93.27 ° C. The specific heat of water is 4.184 J/g × K. What is the specific heat of the metal?
A) 0.670 J/g × K
B) 1.49 J/g × K
C) 0.254 J/g × K
D) 1.03 J/g × K
E) none of these
A) 0.670 J/g × K
B) 1.49 J/g × K
C) 0.254 J/g × K
D) 1.03 J/g × K
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
23
What is the specific heat of an unknown substance if 25.0 grams of the substance absorbs 77.0 calories of heat and the temperature increases by 5.5 ° C?
A) 0.51 cal/g × ° C
B) 0.513 cal/g × ° C
C) 0.56 cal/g × ° C
D) 0.616 cal/g × ° C
E) 0.62 cal/g × ° C
A) 0.51 cal/g × ° C
B) 0.513 cal/g × ° C
C) 0.56 cal/g × ° C
D) 0.616 cal/g × ° C
E) 0.62 cal/g × ° C

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
24
How many calories of heat energy are required to raise the temperature of 25.0 g of silver from 25 ° C tO5 0 ° C?
Specific Heat(Ag) = 0.057 cal/g × ° C
A) 0.057 calories
B) 18 calories
C) 36 calories
D) 48 calories
E) 48.0 calories
Specific Heat(Ag) = 0.057 cal/g × ° C
A) 0.057 calories
B) 18 calories
C) 36 calories
D) 48 calories
E) 48.0 calories

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
25
A chemical reaction releases enough heat to raise the temperature of 77.2 g of water from 24.30 ° C tO45.60 ° C. Given that the specific heat of water is 4.184 J/g × K, what is the heat evolved?
A) 6.02 kJ
B) 4.585 kJ
C) 9.15 kJ
D) 6.88 kJ
E) none of these
A) 6.02 kJ
B) 4.585 kJ
C) 9.15 kJ
D) 6.88 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
26
How much heat must be added to a 4.33 g piece of aluminum to raise its temperature from 22.00 ° C to 27.00 ° C?
Specific Heat(Al) = 0.900 J/g × K
A) 19.5 J
B) 22.8 J
C) 6.88 J
D) 15.2 J
E) none of these
Specific Heat(Al) = 0.900 J/g × K
A) 19.5 J
B) 22.8 J
C) 6.88 J
D) 15.2 J
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
27
When a 60.0 g sample of a metal at 100.0 ° C is added tO45.0 g of water at 22.00 ° C, the final temperature of both the water and metal is 32.81 ° C. The specific heat of water is 4.184 J/g × K. What is the specific heat of the metal?
A) 33.9 J/g × K
B) 0.339 J/g × K
C) 0.505 J/g × K
D) 0.199 J/g × K
E) none of these
A) 33.9 J/g × K
B) 0.339 J/g × K
C) 0.505 J/g × K
D) 0.199 J/g × K
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
28
The reaction of 8.00 g of SO2 (g) burns in excess oxygen. 9.85 kJ of heat are released. What is D H for the following thermochemical equation?
2 SO2 (g) + O2 (g)→2 SO3 (g)
A) - 1.58×102 kJ
B) - 78.8 kJ
C) - 39.4 kJ
D) +78.8 kJ
E) none of these
2 SO2 (g) + O2 (g)→2 SO3 (g)
A) - 1.58×102 kJ
B) - 78.8 kJ
C) - 39.4 kJ
D) +78.8 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
29
When a 50.0 g sample of a metal at 100.0 ° C is added tO60.0 g of water at 25.10 ° C, the final temperature of both the water and metal is 32.81 ° C. The specific heat of water is 4.184 J/g × K. What is the specific heat of the metal?
A) 0.576 J/g × K
B) 1.66 J/g × K
C) 45.0 J/g × K
D) 0.900 J/g × K
E) none of these
A) 0.576 J/g × K
B) 1.66 J/g × K
C) 45.0 J/g × K
D) 0.900 J/g × K
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
30
How many calories of heat are required to heat 52.7 g of Aluminum from 100 ° C to 285 ° C?
Specific Heat(Al) = 0.22 cal/g × ° C
A) 1.3 cal
B) 16 cal
C) 2.1×103 cal
D) 3.3×103 cal
E) 4.4×104 cal
Specific Heat(Al) = 0.22 cal/g × ° C
A) 1.3 cal
B) 16 cal
C) 2.1×103 cal
D) 3.3×103 cal
E) 4.4×104 cal

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
31
When 5.00 g of butane, C4H10 (g), burns in oxygen, 247 kJ of heat are released. What is D H for the following thermochemical equation?
2 C4H10 (g) + 13 O₂(g) 8 CO₂ (g) + 10 H₂ O (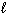 )
)
A) +2.87×103 kJ
B) +5.74×103 kJ
C) - 5.74×103 kJ
D) - 2.87×103 kJ
E) none of these
2 C4H10 (g) + 13 O₂(g) 8 CO₂ (g) + 10 H₂ O (
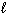 )
)A) +2.87×103 kJ
B) +5.74×103 kJ
C) - 5.74×103 kJ
D) - 2.87×103 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
32
Which statement below is true?
A) Some substances have a negative specific heat.
B) In endothermic reactions, heat is given off to the surroundings.
C) Specific heat is the same for all liquids but metals each have a different specific heat.
D) In exothermic reactions, the sign of D H is always negative.
E) All four of these are false.
A) Some substances have a negative specific heat.
B) In endothermic reactions, heat is given off to the surroundings.
C) Specific heat is the same for all liquids but metals each have a different specific heat.
D) In exothermic reactions, the sign of D H is always negative.
E) All four of these are false.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
33
How much heat must be removed from a 10.0 g sample of graphite (specific heat = 0.720 J/g × K) to decrease its temperature from 55.00 ° C tO44.00 ° C?
A) 0.720 J
B) 7.20 J
C) 7.92 J
D) 79.2 J
E) none of these
A) 0.720 J
B) 7.20 J
C) 7.92 J
D) 79.2 J
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
34
When 10.0 g of ethane, C₂ H₆(g), burns in oxygen, 519 kJ of heat are released. What is D H for the following thermochemical equation?
2 C₂ H₆(g) + 7 O₂(g) 4 CO₂ (g) + 6 H₂ O (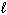 )
)
A) +3.12×103 kJ
B) +1.56×103 kJ
C) - 3.12×103 kJ
D) - 1.56×103 kJ
E) none of these
2 C₂ H₆(g) + 7 O₂(g) 4 CO₂ (g) + 6 H₂ O (
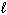 )
)A) +3.12×103 kJ
B) +1.56×103 kJ
C) - 3.12×103 kJ
D) - 1.56×103 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
35
How much heat must be added to a 2.22 g piece of gold (specific heat = 0.129 J/g × K) to raise its temperature from 25.00 ° C to 34.00 ° C?
A) 2.44 J
B) 2.86 J
C) 2.58 J
D) 15.2 J
E) none of these
A) 2.44 J
B) 2.86 J
C) 2.58 J
D) 15.2 J
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
36
A chemical reaction releases enough heat to raise the temperature of 35.2 g of water from 25.60 ° C to 34.80 ° C. Given that the specific heat of water is 4.184 J/g × K, what is the heat evolved?
A) 6.22 kJ
B) 2.55 kJ
C) 9.15 kJ
D) 1.35 kJ
E) none of these
A) 6.22 kJ
B) 2.55 kJ
C) 9.15 kJ
D) 1.35 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
37
The heat exchange of a certain exothermic reaction is measured in a solution calorimeter. The sign convention for the heat exchanged in this process would be a ____ value for the heat released in the reaction and a ____ value for the heat absorbed by the calorimeter.
A) negative ( - ), positive (+)
B) positive (+), negative ( - )
C) negative ( - ), negative ( - )
D) positive (+), positive (+)
A) negative ( - ), positive (+)
B) positive (+), negative ( - )
C) negative ( - ), negative ( - )
D) positive (+), positive (+)

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
38
How much heat is required to raise the temperature of 25.0 grams of water from 32.0 ° C to 87.0 ° C?
Specific Heat (H2O) = 4.184 J/g × ° C
A) 9.20 J
B) 329 J
C) 3.35×103 J
D) 5.75×103 J
E) 1.05×104 J
Specific Heat (H2O) = 4.184 J/g × ° C
A) 9.20 J
B) 329 J
C) 3.35×103 J
D) 5.75×103 J
E) 1.05×104 J

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
39
A reaction of 3.44 g of H2 (g) and 20.5 g of C (s) yields C3H6 (g), and the enthalphy change is - 11.6 kJ. What is D H for the following reaction?
3 H2 (g) + 3 C (s)→C3H6 (g)
A) - 20.4 kJ
B) - 11.6 kJ
C) - 31.7 kJ
D) - 61.2 kJ
E) none of these
3 H2 (g) + 3 C (s)→C3H6 (g)
A) - 20.4 kJ
B) - 11.6 kJ
C) - 31.7 kJ
D) - 61.2 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
40
When a 83.0 g sample of a metal at 100.0 ° C is added tO45.0 g of water at 23.25 ° C, the final temperature of both the water and metal is 27.68 ° C. The specific heat of water is 4.184 J/g × K. What is the specific heat of the metal?
A) 10.0 J/g × K
B) 11.5 J/g × K
C) 0.100 J/g × K
D) 0.139 J/g × K
E) none of these
A) 10.0 J/g × K
B) 11.5 J/g × K
C) 0.100 J/g × K
D) 0.139 J/g × K
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
41
What is the final temperature upon mixing 75.0 grams of water held at 45.0 ° C witH₂ 5.0 grams of water held at 90.0 ° C?
A) 11.3 ° C
B) 28.1 ° C
C) 56.3 ° C
D) 67.5 ° C
E) 78.8 ° C
A) 11.3 ° C
B) 28.1 ° C
C) 56.3 ° C
D) 67.5 ° C
E) 78.8 ° C

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
42
The specific heat of water is 4.184 J/g × ° C. What is the heat capacity of 0.185 kg of liquid water?
A) 774 J/ ° C
B) 44.2 J/ ° C
C) 22.6 J/ ° C
D) 4.184 J/ ° C
E) 0.7774 J/ ° C
A) 774 J/ ° C
B) 44.2 J/ ° C
C) 22.6 J/ ° C
D) 4.184 J/ ° C
E) 0.7774 J/ ° C

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
43
Given the following two equations and their respective enthalpies:
S (s) + O2 (g)→SO2 (g) D H rxn = - 296.8 kJ/mol S (s) + 3/2 O2 (g)→SO3 (g) D H rxn = - 395.6 kJ/mol What is the enthalpy change D H rxn for the following reaction?
2 SO3 (g)→2 SO2 (g) + O2 (g) D H rxn = ???
A) - 692.4 kJ/mol
B) 197.6 kJ/mol
C) 98.8 kJ/mol
D) - 1384.8 kJ/mol
E) None of these
S (s) + O2 (g)→SO2 (g) D H rxn = - 296.8 kJ/mol S (s) + 3/2 O2 (g)→SO3 (g) D H rxn = - 395.6 kJ/mol What is the enthalpy change D H rxn for the following reaction?
2 SO3 (g)→2 SO2 (g) + O2 (g) D H rxn = ???
A) - 692.4 kJ/mol
B) 197.6 kJ/mol
C) 98.8 kJ/mol
D) - 1384.8 kJ/mol
E) None of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
44
Given the following information:
1/2 N2 (g) + 1/2 O2 (g)→NO (g) D H = 90.3 kJ NO (g) + 1/2 Cl2 (g)→NOCl (g) D H = - 38.6 kJ What is the enthalpy of reaction D H rxn for the reaction that follows?
2 NOCl (g)→N2 (g) + O2 (g) + Cl2 (g) D H net rxn = ??
A) D H = - 257.8 kJ
B) D H = - 103.4 kJ
C) D H = - 25.9 kJ
D) D H = - 13.1 kJ
E) D H = 51.7 kJ
1/2 N2 (g) + 1/2 O2 (g)→NO (g) D H = 90.3 kJ NO (g) + 1/2 Cl2 (g)→NOCl (g) D H = - 38.6 kJ What is the enthalpy of reaction D H rxn for the reaction that follows?
2 NOCl (g)→N2 (g) + O2 (g) + Cl2 (g) D H net rxn = ??
A) D H = - 257.8 kJ
B) D H = - 103.4 kJ
C) D H = - 25.9 kJ
D) D H = - 13.1 kJ
E) D H = 51.7 kJ

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
45
Given the equations listed below and their corresponding enthalpies:
N2O4 (g)→2 NO2 (g) D H = 58 kJ 2 NO (g) + O2 (g)→2 NO2 (g) D H = - 112 kJ What is D H for the following reaction?
2 NO (g) + O2 (g)→N2O4 (g)
A) - 170 kJ
B) - 54 kJ
C) - 112 kJ
D) - 2 kJ
E) none of these
N2O4 (g)→2 NO2 (g) D H = 58 kJ 2 NO (g) + O2 (g)→2 NO2 (g) D H = - 112 kJ What is D H for the following reaction?
2 NO (g) + O2 (g)→N2O4 (g)
A) - 170 kJ
B) - 54 kJ
C) - 112 kJ
D) - 2 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following substances would have a Standard Molar Enthalpy of Formation equal to zero, D H ° f = 0?
A) H₂ O (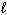 )
)
B) CO2 (g)
C) N2 (g)
D) CH4 (g)
E) O₂(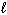 )
)
A) H₂ O (
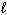 )
)B) CO2 (g)
C) N2 (g)
D) CH4 (g)
E) O₂(
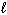 )
)
Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
47
75.0 joules of heat is supplied to ethanol that has a mass of 125 g. What temperature change does this sample of ethanol undergo?
Specific Heat(ethanol) = 2.427 J/g × ° C
A) 0.247 ° C
B) 1.46 ° C
C) 4.05 ° C
D) 3.86×103 ° C
E) 2.28×104 ° C
Specific Heat(ethanol) = 2.427 J/g × ° C
A) 0.247 ° C
B) 1.46 ° C
C) 4.05 ° C
D) 3.86×103 ° C
E) 2.28×104 ° C

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
48
Given the following data:
Fe2O3 (s) + 3 CO (g)→2 Fe (s) + 3 CO2 (g) D H = - 25 kJ/mol 2 CO (g) + O2 (g)→2 CO2 (g) D H = - 566 kJ/mol What is the molar enthalpy of reaction D H rxn for the following reaction?
4 Fe (s) + 3 O2 (g)→2 Fe2O3 (s) D H rxn = ??
A) - 541 kJ/mol
B) - 591 kJ/mol
C) - 1057 kJ/mol
D) - 1648 kJ/mol
E) - 1748 kJ/mol
Fe2O3 (s) + 3 CO (g)→2 Fe (s) + 3 CO2 (g) D H = - 25 kJ/mol 2 CO (g) + O2 (g)→2 CO2 (g) D H = - 566 kJ/mol What is the molar enthalpy of reaction D H rxn for the following reaction?
4 Fe (s) + 3 O2 (g)→2 Fe2O3 (s) D H rxn = ??
A) - 541 kJ/mol
B) - 591 kJ/mol
C) - 1057 kJ/mol
D) - 1648 kJ/mol
E) - 1748 kJ/mol

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
49
A 350 gram aluminum part at an initial temperature of 25.0 ° C absorbs 6200 J of heat. What is the final temperature of the aluminum part?
C(Al) = 0.900 J/g × ° C
A) 5.3 ° C
B) 15.9 ° C
C) 19.7 ° C
D) 40.9 ° C
E) 44.7 ° C
C(Al) = 0.900 J/g × ° C
A) 5.3 ° C
B) 15.9 ° C
C) 19.7 ° C
D) 40.9 ° C
E) 44.7 ° C

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following substances would be expected to have an enthalpy of formation value D H f equal to zero?
(Pay careful attention to the phase indicators.)
I. O₂(g)
II. N₂(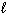 )
)
III. H₂ O (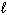 )
)
A) I only
B) I and II
C) I and III
D) II and III
E) All of these
(Pay careful attention to the phase indicators.)
I. O₂(g)
II. N₂(
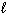 )
)III. H₂ O (
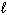 )
)A) I only
B) I and II
C) I and III
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
51
50.0 calories of heat is supplied to a piece of gold of unknown mass. The temperature of the gold ingot raises 15.4 ° C. What is the mass of the gold ingot in grams?
Specific Heat(Au) = 0.0310 cal/g × ° C
A) 9.55×10 - 3 g
B) 0.101 g
C) 9.94 g
D) 23.9 g
E) 105 g
Specific Heat(Au) = 0.0310 cal/g × ° C
A) 9.55×10 - 3 g
B) 0.101 g
C) 9.94 g
D) 23.9 g
E) 105 g

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
52
Given the thermochemical equations and their corresponding enthalpies:
CH4 (g) + 2 O₂(g) CO₂ (g) + 2 H₂ O (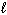 ) D H = - 890 kJ C₂ H4 (g) + 3 O₂(g) 2 CO₂ (g) + 2 H₂ O (
) D H = - 890 kJ C₂ H4 (g) + 3 O₂(g) 2 CO₂ (g) + 2 H₂ O ( 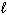 ) D H = - 1410 kJ What is the D H for the following reaction?
) D H = - 1410 kJ What is the D H for the following reaction?
2 CH4 (g) + O₂(g) C₂ H4 (g) + 2 H₂ O (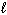 )
)
A) 3190 kJ
B) - 2300 kJ
C) - 520 kJ
D) - 370 kJ
E) none of these
CH4 (g) + 2 O₂(g) CO₂ (g) + 2 H₂ O (
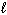 ) D H = - 890 kJ C₂ H4 (g) + 3 O₂(g) 2 CO₂ (g) + 2 H₂ O (
) D H = - 890 kJ C₂ H4 (g) + 3 O₂(g) 2 CO₂ (g) + 2 H₂ O ( 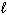 ) D H = - 1410 kJ What is the D H for the following reaction?
) D H = - 1410 kJ What is the D H for the following reaction?2 CH4 (g) + O₂(g) C₂ H4 (g) + 2 H₂ O (
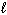 )
)A) 3190 kJ
B) - 2300 kJ
C) - 520 kJ
D) - 370 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following substances is expected to have a standard molar enthalpy of formation D H ° f equal to zero?
A) NaCl (s)
B) H2 (g)
C) HCl (aq)
D) N₂(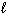 )
)
E) CO2 (s)
A) NaCl (s)
B) H2 (g)
C) HCl (aq)
D) N₂(
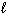 )
)E) CO2 (s)

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
54
When a 2.00 g sample of ammonium nitrate (NH4NO3) dissolves in 48.0 g of water the temperature changes from 26.57 ° C to 23.50 ° C. Assuming the specific heat of the solution is 4.184 J/g × K, what is the enthalpy change for dissolving 2.00 g of ammonium nitrate in water?
A) +616 J
B) +642 J
C) - 642 J
D) - 616 J
E) none of these
A) +616 J
B) +642 J
C) - 642 J
D) - 616 J
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
55
Given the following two equations and their corresponding enthalpies:
CO (g) + 1/2 O2 (g)→CO2 (g) D H 1 = - 283.0 kJ N2 (g) + O2 (g)→2 NO (g) D H 2 = 180.6 kJ What is the enthalpy of reaction D H rxn for the reaction that follows?
CO (g) + NO (g)→CO2 (g) + 1/2 N2 (g) D H net rxn = ??
A) D H rxn = - 746.6 kJ
B) D H rxn = - 385.4 kJ
C) D H rxn = - 373.3 kJ
D) D H rxn = - 192.7 kJ
E) D H rxn = - 102.4 kJ
CO (g) + 1/2 O2 (g)→CO2 (g) D H 1 = - 283.0 kJ N2 (g) + O2 (g)→2 NO (g) D H 2 = 180.6 kJ What is the enthalpy of reaction D H rxn for the reaction that follows?
CO (g) + NO (g)→CO2 (g) + 1/2 N2 (g) D H net rxn = ??
A) D H rxn = - 746.6 kJ
B) D H rxn = - 385.4 kJ
C) D H rxn = - 373.3 kJ
D) D H rxn = - 192.7 kJ
E) D H rxn = - 102.4 kJ

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
56
Given the following thermochemical equations and their corresponding enthalpies:
2 C₂ H₂ (g) + 5 O₂(g) 4 CO₂ (g) + 2 H₂ O (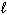 ) D H = - 2601 kJ C8 H8 (
) D H = - 2601 kJ C8 H8 ( 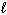 ) + 10 O₂(g) 8 CO₂ (g) + 4 H₂ O (
) + 10 O₂(g) 8 CO₂ (g) + 4 H₂ O ( 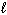 ) D H = - 4393 kJ What is the enthalpy change for the following reaction?
) D H = - 4393 kJ What is the enthalpy change for the following reaction?
4 C₂ H₂ (g) C8 H8 (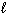 ) D H = ?
) D H = ?
A) - 1792 kJ
B) - 809 kJ
C) - 6994 kJ
D) - 9595 kJ
E) none of these
2 C₂ H₂ (g) + 5 O₂(g) 4 CO₂ (g) + 2 H₂ O (
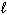 ) D H = - 2601 kJ C8 H8 (
) D H = - 2601 kJ C8 H8 ( 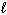 ) + 10 O₂(g) 8 CO₂ (g) + 4 H₂ O (
) + 10 O₂(g) 8 CO₂ (g) + 4 H₂ O ( 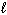 ) D H = - 4393 kJ What is the enthalpy change for the following reaction?
) D H = - 4393 kJ What is the enthalpy change for the following reaction?4 C₂ H₂ (g) C8 H8 (
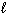 ) D H = ?
) D H = ?A) - 1792 kJ
B) - 809 kJ
C) - 6994 kJ
D) - 9595 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following substances would have a standard enthalpy of formation D H f equal to 0 kJ?
(Pay careful attention to the phase indicators.)
I. H₂ O (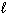 )
)
II. Fe (g)
III. C (s; graphite)
A) I only
B) II only
C) III only
D) I and III
E) II and III
(Pay careful attention to the phase indicators.)
I. H₂ O (
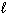 )
)II. Fe (g)
III. C (s; graphite)
A) I only
B) II only
C) III only
D) I and III
E) II and III

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
58
Which unit from those listed below would be the best choice as a unit for the heat capacity of a substance?
A) J
B) J/mol
C) J/g
D) J/ ° C
E) J/g × ° C
A) J
B) J/mol
C) J/g
D) J/ ° C
E) J/g × ° C

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
59
Given the equations shown below and their corresponding enthalpies:
H₂ O₂ (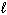 ) H₂ O (
) H₂ O ( 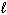 ) + 1 / 2 O₂(g) D H = - 98 kJ H₂ (g) + 1 / 2 O₂(g) H₂ O (
) + 1 / 2 O₂(g) D H = - 98 kJ H₂ (g) + 1 / 2 O₂(g) H₂ O ( 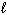 ) D H = - 285 kJ What is D H for the following reaction?
) D H = - 285 kJ What is D H for the following reaction?
H₂ O₂ (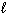 ) H₂ (g) + O₂(g)
) H₂ (g) + O₂(g)
A) - 383 kJ
B) - 187 kJ
C) 383 kJ
D) 187 kJ
E) none of these
H₂ O₂ (
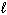 ) H₂ O (
) H₂ O ( 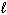 ) + 1 / 2 O₂(g) D H = - 98 kJ H₂ (g) + 1 / 2 O₂(g) H₂ O (
) + 1 / 2 O₂(g) D H = - 98 kJ H₂ (g) + 1 / 2 O₂(g) H₂ O ( 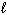 ) D H = - 285 kJ What is D H for the following reaction?
) D H = - 285 kJ What is D H for the following reaction?H₂ O₂ (
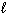 ) H₂ (g) + O₂(g)
) H₂ (g) + O₂(g)A) - 383 kJ
B) - 187 kJ
C) 383 kJ
D) 187 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
60
In a calorimetry experiment, 0.500 g of copper dissolved in water that contains bromine, 1382 joules of heat were released to the surroundings. What is D H for the following thermochemical equation?
Cu (s) + Br2 (aq)→CuBr2 (aq) D H = ?
A) - 2.76 kJ
B) +2.76 kJ
C) - 176 kJ
D) - 351 kJ
E) none of these
Cu (s) + Br2 (aq)→CuBr2 (aq) D H = ?
A) - 2.76 kJ
B) +2.76 kJ
C) - 176 kJ
D) - 351 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
61
Given the data below, what is D H for the following reaction?
2 CH4 (g) + 3 O₂(g) 2 CO (g) + 4 H₂ O (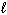 ) D H f CH4 (g) = - 75 kJ/mol D H f CO (g) = - 110 kJ/mol D H f H₂ O (
) D H f CH4 (g) = - 75 kJ/mol D H f CO (g) = - 110 kJ/mol D H f H₂ O ( 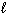 ) = - 286 kJ/mol
) = - 286 kJ/mol
A) - 1214 kJ
B) - 1514 kJ
C) - 321 kJ
D) - 842 kJ
E) none of these
2 CH4 (g) + 3 O₂(g) 2 CO (g) + 4 H₂ O (
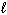 ) D H f CH4 (g) = - 75 kJ/mol D H f CO (g) = - 110 kJ/mol D H f H₂ O (
) D H f CH4 (g) = - 75 kJ/mol D H f CO (g) = - 110 kJ/mol D H f H₂ O ( 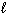 ) = - 286 kJ/mol
) = - 286 kJ/molA) - 1214 kJ
B) - 1514 kJ
C) - 321 kJ
D) - 842 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
62
Given the data below, what is D H for the following reaction?
2 H₂ S (g) + 3 O₂(g) 2 H₂ O (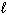 ) + 2 SO₂ (g) D H f H₂ S (g) = - 21 kJ/mol D H f H₂ O (
) + 2 SO₂ (g) D H f H₂ S (g) = - 21 kJ/mol D H f H₂ O ( 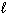 ) = - 286 kJ/mol D H f SO₂ (g) = - 297 kJ/mol
) = - 286 kJ/mol D H f SO₂ (g) = - 297 kJ/mol
A) - 562 kJ
B) 832 kJ
C) - 1124 kJ
D) - 1420 kJ
E) none of these
2 H₂ S (g) + 3 O₂(g) 2 H₂ O (
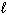 ) + 2 SO₂ (g) D H f H₂ S (g) = - 21 kJ/mol D H f H₂ O (
) + 2 SO₂ (g) D H f H₂ S (g) = - 21 kJ/mol D H f H₂ O ( 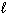 ) = - 286 kJ/mol D H f SO₂ (g) = - 297 kJ/mol
) = - 286 kJ/mol D H f SO₂ (g) = - 297 kJ/molA) - 562 kJ
B) 832 kJ
C) - 1124 kJ
D) - 1420 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
63
What is the enthalpy of reaction D H rxn for the following reaction?
SiO₂ (s) + 4 HF (g) SiF4 (g) + 2 H₂ O (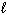 ) D H rxn = ??
) D H rxn = ??
D H f (SiO₂ ) = - 910.9 kJ/mol D H f (HF) = - 273.0 kJ/mol D H f (SiF4 ) = - 1614.9 kJ/mol D H f (H₂ O) = - 285.8 kJ/mol
A) - 183.6 kJ/mol
B) - 716.8 kJ/mol
C) - 1002.6 kJ/mol
D) - 3084.6 kJ/mol
E) - 4189.4 kJ/mol
SiO₂ (s) + 4 HF (g) SiF4 (g) + 2 H₂ O (
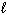 ) D H rxn = ??
) D H rxn = ??D H f (SiO₂ ) = - 910.9 kJ/mol D H f (HF) = - 273.0 kJ/mol D H f (SiF4 ) = - 1614.9 kJ/mol D H f (H₂ O) = - 285.8 kJ/mol
A) - 183.6 kJ/mol
B) - 716.8 kJ/mol
C) - 1002.6 kJ/mol
D) - 3084.6 kJ/mol
E) - 4189.4 kJ/mol

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
64
Given the standard heats of formation listed below, what is D H for the following reaction?
C6 H12 O6 (s) + 6 O₂(g) 6 CO₂ (g) + 6 H₂ O (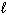 ) D H f C6 H12 O6 (s) = - 1268 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O (
) D H f C6 H12 O6 (s) = - 1268 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O ( 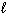 ) = - 286 kJ/mol
) = - 286 kJ/mol
A) - 5332 kJ
B) +588 kJ
C) - 2812 kJ
D) None of these answers are correct (although the problem can be worked with the data given).
E) Not enough information is given to work the problem.
C6 H12 O6 (s) + 6 O₂(g) 6 CO₂ (g) + 6 H₂ O (
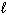 ) D H f C6 H12 O6 (s) = - 1268 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O (
) D H f C6 H12 O6 (s) = - 1268 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O ( 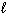 ) = - 286 kJ/mol
) = - 286 kJ/molA) - 5332 kJ
B) +588 kJ
C) - 2812 kJ
D) None of these answers are correct (although the problem can be worked with the data given).
E) Not enough information is given to work the problem.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the equations below corresponds to the standard molar enthalpy of formation for AgNO3, D H f(AgNO3)?
A) Ag (s) + 1/2 N2 (g) + 3/2 O2 (g)→ AgNO3 (s)
B) Ag+1 (aq) + NO3 - 1 (aq)→ AgNO3 (s)
C) Ag (s) + N (g) + O3 (g)→ AgNO3 (s)
D) 2 Ag (s) + N2 (g) + 3 O2 (g)→ 2 AgNO3 (s)
A) Ag (s) + 1/2 N2 (g) + 3/2 O2 (g)→ AgNO3 (s)
B) Ag+1 (aq) + NO3 - 1 (aq)→ AgNO3 (s)
C) Ag (s) + N (g) + O3 (g)→ AgNO3 (s)
D) 2 Ag (s) + N2 (g) + 3 O2 (g)→ 2 AgNO3 (s)

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
66
For which of the equations shown below does the standard enthalpy of reaction equal the standard molar enthalpy of reaction of the product?
I. 1 / 2 N₂(g) + 3 / 2 H₂ (g) NH₃(g)
II. H₂ (g) + Br₂(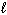 ) 2 HBr (g)
) 2 HBr (g)
III. HCl (g) + NH₃(g) NH ₄Cl (s)
A) I only
B) II only
C) III only
D) I and II
E) I and III
I. 1 / 2 N₂(g) + 3 / 2 H₂ (g) NH₃(g)
II. H₂ (g) + Br₂(
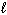 ) 2 HBr (g)
) 2 HBr (g)III. HCl (g) + NH₃(g) NH ₄Cl (s)
A) I only
B) II only
C) III only
D) I and II
E) I and III

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following defines the heat of formation of Cr2O3 (s)?
A) 2 Cr (s) + 3 O (g)→ Cr2O3 (s)
B) 2 Cr (s) + 3/2 O2 (g)→ Cr2O3 (s)
C) 4 Cr (s) + 3 O2 (g)→ 2 Cr2O3 (s)
D) Cr2 (s) + 3/2 O2 (g)→ Cr2O3 (s)
E) none of these
A) 2 Cr (s) + 3 O (g)→ Cr2O3 (s)
B) 2 Cr (s) + 3/2 O2 (g)→ Cr2O3 (s)
C) 4 Cr (s) + 3 O2 (g)→ 2 Cr2O3 (s)
D) Cr2 (s) + 3/2 O2 (g)→ Cr2O3 (s)
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
68
Given the following Thermodynamic data:
D H f (Fe2 O3 ; s) = - 822 kJ/mol D H f (FeCl 3 ; s) = - 400 kJ/mol D H f (H₂ O; g) = - 242 kJ/mol D H f (H₂ O;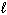 ) = - 286 kJ/mol D H f (HCl; g) = - 92 kJ/mol D H f (HCl; aq) = - 167 kJ/mol What is the Standard Molar Enthalpy of Reaction D H rxn for the following reaction?
) = - 286 kJ/mol D H f (HCl; g) = - 92 kJ/mol D H f (HCl; aq) = - 167 kJ/mol What is the Standard Molar Enthalpy of Reaction D H rxn for the following reaction?
Fe2 O3(s) + 6 HCl (g) 2 FeCl 3 (s) + 3 H₂ O (g) D H rxn = ??
A) - 1256 kJ/mol
B) - 284 kJ/mol
C) - 152 kJ/mol
D) 272 kJ/mol
E) 298 kJ/mol
D H f (Fe2 O3 ; s) = - 822 kJ/mol D H f (FeCl 3 ; s) = - 400 kJ/mol D H f (H₂ O; g) = - 242 kJ/mol D H f (H₂ O;
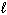 ) = - 286 kJ/mol D H f (HCl; g) = - 92 kJ/mol D H f (HCl; aq) = - 167 kJ/mol What is the Standard Molar Enthalpy of Reaction D H rxn for the following reaction?
) = - 286 kJ/mol D H f (HCl; g) = - 92 kJ/mol D H f (HCl; aq) = - 167 kJ/mol What is the Standard Molar Enthalpy of Reaction D H rxn for the following reaction?Fe2 O3(s) + 6 HCl (g) 2 FeCl 3 (s) + 3 H₂ O (g) D H rxn = ??
A) - 1256 kJ/mol
B) - 284 kJ/mol
C) - 152 kJ/mol
D) 272 kJ/mol
E) 298 kJ/mol

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
69
Given the data below, what is D H for the following reaction?
CH4 (g) + 2 O₂(g) CO₂ (g) + 2 H₂ O (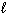 ) D H f CH4 (g) = - 75 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O (
) D H f CH4 (g) = - 75 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O ( 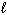 ) = - 286 kJ/mol
) = - 286 kJ/mol
A) - 891 kJ
B) - 605 kJ
C) +605 kJ
D) +891 kJ
E) none of these
CH4 (g) + 2 O₂(g) CO₂ (g) + 2 H₂ O (
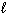 ) D H f CH4 (g) = - 75 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O (
) D H f CH4 (g) = - 75 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O ( 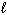 ) = - 286 kJ/mol
) = - 286 kJ/molA) - 891 kJ
B) - 605 kJ
C) +605 kJ
D) +891 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
70
For which of the equations shown below does the standard enthalpy of reaction equal the standard molar enthalpy of reaction of the product?
I. N₂(g) + O₂(g) 2 NO (g)
II. C (s; graphite) + O₂(g) CO₂ (g)
III. H₂ O (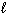 ) + CO₂ (g) H₂ CO3(
) + CO₂ (g) H₂ CO3( 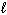 )
)
A) I only
B) II only
C) III only
D) II and III
E) All of these
I. N₂(g) + O₂(g) 2 NO (g)
II. C (s; graphite) + O₂(g) CO₂ (g)
III. H₂ O (
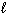 ) + CO₂ (g) H₂ CO3(
) + CO₂ (g) H₂ CO3( 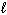 )
)A) I only
B) II only
C) III only
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
71
What is the enthalpy of reaction D H ° rxn for the following reaction?
2 C2H6 (g) + 7 O2 (g)→4 CO2 (g) + 6 H2O (g) D H ° rxn = ??
D H ° f(C2H6) = - 84.7 kJ/mol D H ° f(CO2) = - 393.5 kJ/mol D H ° f(H2O) = - 241.8 kJ/mol
A) - 3194.2 kJ/mol
B) - 2855.4 kJ/mol
C) - 1674.9 kJ/mol
D) - 1646.4 kJ/mol
E) - 550.6 kJ/mol
2 C2H6 (g) + 7 O2 (g)→4 CO2 (g) + 6 H2O (g) D H ° rxn = ??
D H ° f(C2H6) = - 84.7 kJ/mol D H ° f(CO2) = - 393.5 kJ/mol D H ° f(H2O) = - 241.8 kJ/mol
A) - 3194.2 kJ/mol
B) - 2855.4 kJ/mol
C) - 1674.9 kJ/mol
D) - 1646.4 kJ/mol
E) - 550.6 kJ/mol

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following defines the heat of formation of Fe2O3 (s)?
A) 2 Fe (g) + 3 O (g)→ Fe2O3 (s)
B) 2 Fe (s) + 3/2 O2 (g)→ Fe2O3 (s)
C) 4 Fe (s) + 2 O3 (g)→ 2 Fe2O3 (s)
D) 2 Fe (g) + 3 O (g)→ Fe2O3 (g)
E) none of these
A) 2 Fe (g) + 3 O (g)→ Fe2O3 (s)
B) 2 Fe (s) + 3/2 O2 (g)→ Fe2O3 (s)
C) 4 Fe (s) + 2 O3 (g)→ 2 Fe2O3 (s)
D) 2 Fe (g) + 3 O (g)→ Fe2O3 (g)
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
73
What is the standard molar enthalpy change D H rxn for the following reactions using the standard molar enthalpies of formations provided?
SiCl 4 (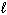 ) + 2 H₂ O (
) + 2 H₂ O ( 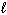 ) SiO₂ (s) + 4 HCl (g) D H rxn = ???
) SiO₂ (s) + 4 HCl (g) D H rxn = ???
D H f (SiCl 4 ) = - 640.1 kJ/mol D H f (SiO₂ ) = - 910.9 kJ/mol D H f (H₂ O) = - 285.8 kJ/mol D H f (HCl) = - 92.3 kJ/mol
A) - 2491.8 kJ/mol
B) - 1929.1 kJ/mol
C) - 77.3 kJ/mol
D) - 68.4 kJ/mol
E) None of these
SiCl 4 (
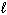 ) + 2 H₂ O (
) + 2 H₂ O ( 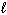 ) SiO₂ (s) + 4 HCl (g) D H rxn = ???
) SiO₂ (s) + 4 HCl (g) D H rxn = ???D H f (SiCl 4 ) = - 640.1 kJ/mol D H f (SiO₂ ) = - 910.9 kJ/mol D H f (H₂ O) = - 285.8 kJ/mol D H f (HCl) = - 92.3 kJ/mol
A) - 2491.8 kJ/mol
B) - 1929.1 kJ/mol
C) - 77.3 kJ/mol
D) - 68.4 kJ/mol
E) None of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
74
Given the following experimental data:
D H rxn Ca (s) + 2 H₂ O (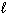 ) Ca(OH) 2 (s) + H₂ (g) - 415.1 kJ 1 / 2 H₂ (g) + 1 / 2 Cl₂ 2 HCl (g) - 92.31 kJ CaCl₂ (s) + 2 H₂ O (
) Ca(OH) 2 (s) + H₂ (g) - 415.1 kJ 1 / 2 H₂ (g) + 1 / 2 Cl₂ 2 HCl (g) - 92.31 kJ CaCl₂ (s) + 2 H₂ O ( 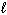 ) Ca(OH) 2 (s) + 2 HCl (g) 195.3 kJ What is the standard heat of formation ( D H f ) for CaCl₂ (s)?
) Ca(OH) 2 (s) + 2 HCl (g) 195.3 kJ What is the standard heat of formation ( D H f ) for CaCl₂ (s)?
A) - 795.0 kJ/mol
B) - 311.3 kJ/mol
C) - 116.8 kJ/mol
D) +127.5 kJ/mol
E) +702.6 kJ/mol
D H rxn Ca (s) + 2 H₂ O (
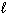 ) Ca(OH) 2 (s) + H₂ (g) - 415.1 kJ 1 / 2 H₂ (g) + 1 / 2 Cl₂ 2 HCl (g) - 92.31 kJ CaCl₂ (s) + 2 H₂ O (
) Ca(OH) 2 (s) + H₂ (g) - 415.1 kJ 1 / 2 H₂ (g) + 1 / 2 Cl₂ 2 HCl (g) - 92.31 kJ CaCl₂ (s) + 2 H₂ O ( 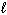 ) Ca(OH) 2 (s) + 2 HCl (g) 195.3 kJ What is the standard heat of formation ( D H f ) for CaCl₂ (s)?
) Ca(OH) 2 (s) + 2 HCl (g) 195.3 kJ What is the standard heat of formation ( D H f ) for CaCl₂ (s)?A) - 795.0 kJ/mol
B) - 311.3 kJ/mol
C) - 116.8 kJ/mol
D) +127.5 kJ/mol
E) +702.6 kJ/mol

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
75
Given the data below, what is D H for the following reaction?
C₂ H5 OH (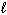 ) + 3 O₂(g) 2 CO₂ (g) + 3 H₂ O (
) + 3 O₂(g) 2 CO₂ (g) + 3 H₂ O ( 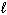 ) D H f C₂ H5 OH (
) D H f C₂ H5 OH ( 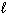 ) = - 278 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O (
) = - 278 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O ( 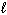 ) = - 286 kJ/mol
) = - 286 kJ/mol
A) - 1368 kJ
B) - 401 kJ
C) +1368 kJ
D) +402 kJ
E) none of these
C₂ H5 OH (
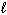 ) + 3 O₂(g) 2 CO₂ (g) + 3 H₂ O (
) + 3 O₂(g) 2 CO₂ (g) + 3 H₂ O ( 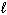 ) D H f C₂ H5 OH (
) D H f C₂ H5 OH ( 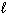 ) = - 278 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O (
) = - 278 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O ( 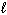 ) = - 286 kJ/mol
) = - 286 kJ/molA) - 1368 kJ
B) - 401 kJ
C) +1368 kJ
D) +402 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
76
Given the data below, what is D H for the following reaction?
4 NO (g) + 6 H₂ O (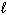 ) 4 NH₃(g) + 5 O₂(g) D H f H₂ O (
) 4 NH₃(g) + 5 O₂(g) D H f H₂ O ( 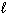 ) = - 286 kJ/mol D H f NH₃(g) = - 46 kJ/mol D H f NO (g) = 90 kJ/mol
) = - 286 kJ/mol D H f NH₃(g) = - 46 kJ/mol D H f NO (g) = 90 kJ/mol
A) +1172 kJ
B) +149 KJ
C) - 149 kJ
D) - 1172 kJ
E) none of these
4 NO (g) + 6 H₂ O (
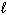 ) 4 NH₃(g) + 5 O₂(g) D H f H₂ O (
) 4 NH₃(g) + 5 O₂(g) D H f H₂ O ( 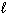 ) = - 286 kJ/mol D H f NH₃(g) = - 46 kJ/mol D H f NO (g) = 90 kJ/mol
) = - 286 kJ/mol D H f NH₃(g) = - 46 kJ/mol D H f NO (g) = 90 kJ/molA) +1172 kJ
B) +149 KJ
C) - 149 kJ
D) - 1172 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
77
Given the data below, what is D H ° for the following reaction?
CH4 (g) + 3 Cl2 (g)→CHCl3 (g) + 3 HCl (g) D H ° f [CH4 (g)] = - 75 kJ/mol D H ° f [CHCl3 (g)] = - 103 kJ/mol D H ° f [HCl (g)] = - 92 kJ/mol
A) - 304 kJ
B) - 120 kJ
C) +304 kJ
D) +120 kJ
E) none of these
CH4 (g) + 3 Cl2 (g)→CHCl3 (g) + 3 HCl (g) D H ° f [CH4 (g)] = - 75 kJ/mol D H ° f [CHCl3 (g)] = - 103 kJ/mol D H ° f [HCl (g)] = - 92 kJ/mol
A) - 304 kJ
B) - 120 kJ
C) +304 kJ
D) +120 kJ
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the equations listed below corresponds to the standard molar enthalpy of formation D H ° f for NH4NO3?
A) NH4+ (aq) + NO3 - (aq)→ NH4NO3 (s)
B) NH3 (g) + HNO3 (g)→ NH4NO3 (s)
C) N2 (g) + 2 H2 (g) + 3/2 O2 (g)→ NH4NO3 (s)
D) 2 N2 (g) + 4 H2 (g) + 3 O2 (g)→ 2 NH4NO3 (s)
E) 2 N (g) + 4 H (g) + 3 O (g)→ NH4NO3 (s)
A) NH4+ (aq) + NO3 - (aq)→ NH4NO3 (s)
B) NH3 (g) + HNO3 (g)→ NH4NO3 (s)
C) N2 (g) + 2 H2 (g) + 3/2 O2 (g)→ NH4NO3 (s)
D) 2 N2 (g) + 4 H2 (g) + 3 O2 (g)→ 2 NH4NO3 (s)
E) 2 N (g) + 4 H (g) + 3 O (g)→ NH4NO3 (s)

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck


