Deck 6: The Gaseous State
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/140
Play
Full screen (f)
Deck 6: The Gaseous State
1
What is the pressure in millimeters of mercury that is equivalent tO5 00 psi?
(1 atm = 760 mm Hg and 1 atm = 14.7 psi)
A) 0.103 mm Hg
B) 9.67 mm Hg
C) 22.3 mm Hg
D) 2.59×104 mm Hg
E) 5.59×106 mm Hg
(1 atm = 760 mm Hg and 1 atm = 14.7 psi)
A) 0.103 mm Hg
B) 9.67 mm Hg
C) 22.3 mm Hg
D) 2.59×104 mm Hg
E) 5.59×106 mm Hg
2.59×104 mm Hg
2
The pressure for inflating a certain automobile tire is 30.0 pounds per square inch (psi). What is this pressure reported in mm of Hg?
(1 atm = 14.7 psi; 760 mm Hg = 1 atm)
A) 2.98×10 - 6 mm Hg
B) 2.69×10 - 3 mm Hg
C) 0.580 mm Hg
D) 1.55×103 mm Hg
E) 3.35×105 mm Hg
(1 atm = 14.7 psi; 760 mm Hg = 1 atm)
A) 2.98×10 - 6 mm Hg
B) 2.69×10 - 3 mm Hg
C) 0.580 mm Hg
D) 1.55×103 mm Hg
E) 3.35×105 mm Hg
1.55×103 mm Hg
3
A certain automobile tire is inflated to 36.0 pounds per square inch (psi). What pressure is this tire reported in mm of Hg?
(1 atm = 14.7 psi and 1 atm = 760 mm Hg)
A) 0.696 mm of Hg
B) 1.44 mm of Hg
C) 2.45 mm of Hg
D) 1.86×103 mm of Hg
E) 4.02×105 of Hg
(1 atm = 14.7 psi and 1 atm = 760 mm Hg)
A) 0.696 mm of Hg
B) 1.44 mm of Hg
C) 2.45 mm of Hg
D) 1.86×103 mm of Hg
E) 4.02×105 of Hg
1.86×103 mm of Hg
4
Express a pressure of 724 torr in atm.
A) 0.724 atm
B) 0.853 atm
C) 0.953 atm
D) 1.05 atm
E) none of these
A) 0.724 atm
B) 0.853 atm
C) 0.953 atm
D) 1.05 atm
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following three states of matter have(has) forces of repulsion that are far greater than their forces of attraction?
I. Solids
II. Liquids
III. Gases
A) III only
B) I and II
C) I and III
D) II and III
E) All of these
I. Solids
II. Liquids
III. Gases
A) III only
B) I and II
C) I and III
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
6
At the peak of Mt. Everest, atmospheric pressure is 220. mm Hg. What is this pressure in pounds per square inch (psi)?
(1 atm = 760 mm Hg and 1 atm = 14.7 psi)
A) 1.97×10 - 2 psi
B) 0.235 psi
C) 4.26 psi
D) 50.8 psi
E) 2.46×106 psi
(1 atm = 760 mm Hg and 1 atm = 14.7 psi)
A) 1.97×10 - 2 psi
B) 0.235 psi
C) 4.26 psi
D) 50.8 psi
E) 2.46×106 psi

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
7
Express a pressure of 0.567 atm in torr.
A) 431 torr
B) 245 torr
C) 563 torr
D) 684 torr
E) none of these
A) 431 torr
B) 245 torr
C) 563 torr
D) 684 torr
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following three states of matter have(has) a definite shape ?
I. Solids
II. Liquids
III. Gases
A) I only
B) I and II
C) I and III
D) II and III
E) All of these
I. Solids
II. Liquids
III. Gases
A) I only
B) I and II
C) I and III
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
9
What are the molar volume, temperature and pressure respectively for an ideal gas at "Standard Temperature and Pressure", STP?
A) 1.0 L, 0 ° C, and 1 atm
B) 1.0 L, 25 ° C and 1 atm
C) 22.4 L, 0 ° C and 1 atm
D) 22.4 L, 25 ° C and 1 atm
E) 22.4 L, 25 ° C and 760 atm
A) 1.0 L, 0 ° C, and 1 atm
B) 1.0 L, 25 ° C and 1 atm
C) 22.4 L, 0 ° C and 1 atm
D) 22.4 L, 25 ° C and 1 atm
E) 22.4 L, 25 ° C and 760 atm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
10
Express a pressure of 1.22 atm in kPa. (1 atm = 1.01×105 Pa)
A) 1.44×103 kPa
B) 124 kPa
C) 24.2 kPa
D) 213 kPa
E) none of these
A) 1.44×103 kPa
B) 124 kPa
C) 24.2 kPa
D) 213 kPa
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
11
Given the three statements below, pick the correct answer.
I. Pressure is directly proportional to temperature in kelvins.
II. Volume is directly proportional to pressure.
III. Volume is directly proportional to the number of moles of the gas sample.
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) all three are true
E) only III is true
I. Pressure is directly proportional to temperature in kelvins.
II. Volume is directly proportional to pressure.
III. Volume is directly proportional to the number of moles of the gas sample.
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) all three are true
E) only III is true

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
12
A gas can be characterized by its volume . Which of the following parameters listed below are indirectly related to the volume of an ideal gas?
I. Pressure (if the mole amount and temperature are held constant).
II. Mole amount of gas (if the pressure and temperature are held constant).
III. Temperature (if the pressure and the mole amount are held constant).
A) I only
B) II only
C) III only
D) II and III
E) All of these
I. Pressure (if the mole amount and temperature are held constant).
II. Mole amount of gas (if the pressure and temperature are held constant).
III. Temperature (if the pressure and the mole amount are held constant).
A) I only
B) II only
C) III only
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
13
The pressure of an ideal gas is directly proportional to which of the following three parameters?
I. Volume of an ideal gas at constant mole amount and temperature
II. Mole amount of an ideal gas at constant volume and temperature
III. Temperature of an ideal gas at constant volume and mole amount
A) II only
B) I and II
C) I and III
D) II and III
E) All of these
I. Volume of an ideal gas at constant mole amount and temperature
II. Mole amount of an ideal gas at constant volume and temperature
III. Temperature of an ideal gas at constant volume and mole amount
A) II only
B) I and II
C) I and III
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
14
A sample of a gas at 2.31 atm of pressure occupies 4.10 L. What will the volume be if the pressure is changed to 1.02 atm at constant temperature?
A) 4.65 L
B) 1.81 L
C) 18.6 L
D) 7.11 L
E) 9.28 L
A) 4.65 L
B) 1.81 L
C) 18.6 L
D) 7.11 L
E) 9.28 L

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
15
Atmospheric pressure on a particular day supports 29.9 inches of Hg. What is this pressure in atm's?
(1 inch Hg = 2.54 cm Hg; 1 atm = 760 mm Hg)
A) 0.0393 atm
B) 0.999 atm
C) 440 atm
D) 760 atm
E) None of these
(1 inch Hg = 2.54 cm Hg; 1 atm = 760 mm Hg)
A) 0.0393 atm
B) 0.999 atm
C) 440 atm
D) 760 atm
E) None of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following units of pressure is equivalent to a "torr"?
A) atm
B) mm of Hg
C) bar
D) psi
E) pascal
A) atm
B) mm of Hg
C) bar
D) psi
E) pascal

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
17
A basketball is inflated to 23.0 psi. How many millimeters of mercury can be supported by this pressure?
(1 atm = 14.7 psi and 1 atm = 760 mm of Hg)
A) 2.06×10 - 3 mm of Hg
B) 4.45×10 - 1 mm of Hg
C) 1.56 mm of Hg
D) 1.19×103 mm of Hg
E) 2.57×105 mm of Hg
(1 atm = 14.7 psi and 1 atm = 760 mm of Hg)
A) 2.06×10 - 3 mm of Hg
B) 4.45×10 - 1 mm of Hg
C) 1.56 mm of Hg
D) 1.19×103 mm of Hg
E) 2.57×105 mm of Hg

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
18
Which property(ies) listed below is(are) consistent with the gaseous state of matter ?
I. Gases have a lower density than liquids or solids and their densities change to a large extent as the temperature changes.
II. Gases are easily compressed to smaller volumes.
III. In general, most gases do not expand upon heating.
A) III only
B) I and II
C) I and III
D) II and III
E) All of these.
I. Gases have a lower density than liquids or solids and their densities change to a large extent as the temperature changes.
II. Gases are easily compressed to smaller volumes.
III. In general, most gases do not expand upon heating.
A) III only
B) I and II
C) I and III
D) II and III
E) All of these.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
19
A gas is initially contained in a moveable cylinder that occupies a volume of 900. mL under a pressure of 3.00 atm. What would be the volume of this same gas if the pressure is reduced to 0.500 atm at constant temperature?
A) 1.86×10 - 4 mL
B) 150 mL
C) 600 mL
D) 1.35×103 mL
E) 5.40×103 mL
A) 1.86×10 - 4 mL
B) 150 mL
C) 600 mL
D) 1.35×103 mL
E) 5.40×103 mL

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
20
A "Condensed phase" refers to what?
A) gases only
B) gases and liquids
C) liquids only
D) liquids and solids
E) solids only
A) gases only
B) gases and liquids
C) liquids only
D) liquids and solids
E) solids only

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
21
A 5.0 mol sample of gas exerts a pressure of 2.0 atm at 27 ° C in a certain container. What would be the pressure inside this container if 2.5 mol of the same gas are present at a temperature of 100 ° C?
A) 0.80 atm
B) 1.6 atm
C) 1.2 atm
D) 3.7 atm
E) none of these
A) 0.80 atm
B) 1.6 atm
C) 1.2 atm
D) 3.7 atm
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
22
A sample of N2 gas occupies a volume of 375 mL at 25 ° C and a pressure of 2.0 atm. What is the temperature in degrees Celsius of this sample of N2 gas when it occupies a volume of 525 mL at the same pressure?
A) 35 ° C
B) 144 ° C
C) 194 ° C
D) 417 ° C
E) 690 ° C
A) 35 ° C
B) 144 ° C
C) 194 ° C
D) 417 ° C
E) 690 ° C

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
23
What is the final pressure of a gas if a given sample at 300 ° C, 2.2 atm and 2.4 L is changed to 0 ° C and 1.0 L?
A) 6.4 atm
B) 9.1 atm
C) 2.5 atm
D) 5.3 atm
E) none of these
A) 6.4 atm
B) 9.1 atm
C) 2.5 atm
D) 5.3 atm
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
24
At constant temperature, a sample of 6.0 L of O2 at 3.0 atm pressure is compressed until the volume decreases to 2.5 L. What is the new pressure in atmospheres?
A) 0.14 atm
B) 1.3 atm
C) 5.0 atm
D) 7.2 atm
E) 45 atm
A) 0.14 atm
B) 1.3 atm
C) 5.0 atm
D) 7.2 atm
E) 45 atm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
25
What pressure will a given sample of gas be at 27 ° C if at 100 ° C this gas exerts a pressure oF4.0 atm, all measurements at constant volume?
A) 5.0 atm
B) 3.2 atm
C) 1.1 atm
D) 15 atm
E) none of these
A) 5.0 atm
B) 3.2 atm
C) 1.1 atm
D) 15 atm
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
26
A sample of gas at 1.3 atm of pressure is in a 2.0 L container. What will the pressure be if the sample is transferred to a 0.90 L container?
A) 0.59 atm
B) 1.4 atm
C) 2.9 atm
D) 3.2 atm
E) none of these
A) 0.59 atm
B) 1.4 atm
C) 2.9 atm
D) 3.2 atm
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
27
A balloon is inflated to a volume of 5.0 L at 25 ° C and 1.00 atm. At what temperature will the volume of the balloon reacH₆.0 L if the pressure is not changed?
A) - 25 ° C
B) 21 ° C
C) 30 ° C
D) 85 ° C
E) none of these
A) - 25 ° C
B) 21 ° C
C) 30 ° C
D) 85 ° C
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
28
A sample of SF6 gas occupies a volume of 5.01 L at 198 ° C. Assuming the pressure is constant, what temperature in ° C is needed to reduce the volume to 2.24 L?
A) - 62.6 ° C
B) 88.5 ° C
C) 211 ° C
D) 443 ° C
E) 780 ° C
A) - 62.6 ° C
B) 88.5 ° C
C) 211 ° C
D) 443 ° C
E) 780 ° C

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
29
A sample of gaseous anesthetic cyclopropane, with a volume oF425 mL at a temperature of 27 ° C is compressed tO415 mL at constant pressure by cooling the gas. What is the new temperature in degrees Celsius at this volume?
A) 10 ° C
B) 20 ° C
C) 28 ° C
D) 34 ° C
E) 293 ° C
A) 10 ° C
B) 20 ° C
C) 28 ° C
D) 34 ° C
E) 293 ° C

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
30
A sample of Argon gas occupies a volume of 1.20 L at 125 ° C and 1 atm. What would be the temperature in degrees Celsius for this same gas, if its volume is increased tO5 .00 L at the same pressure?
A) - 177 ° C
B) 521 ° C
C) 1.39×103 ° C
D) 1.66×103 ° C
E) 1.93×103 ° C
A) - 177 ° C
B) 521 ° C
C) 1.39×103 ° C
D) 1.66×103 ° C
E) 1.93×103 ° C

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
31
If the volume of an ideal gas is 2.0 L at 0.50 atm of pressure, what is the final pressure if the volume increases tO5 .0 L?
A) 0.20 atm
B) 1.3 atm
C) 1.0 atm
D) 0.50 atm
E) none of these
A) 0.20 atm
B) 1.3 atm
C) 1.0 atm
D) 0.50 atm
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
32
What volume will a sample of gas occupy at 400 ° C if at 100 ° C it occupies 420 mL (pressure is constant)?
A) 233 mL
B) 1.26×102 mL
C) 582 mL
D) 758 mL
E) none of these
A) 233 mL
B) 1.26×102 mL
C) 582 mL
D) 758 mL
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
33
If an ideal gas occupies 2.0 L at a temperature of 27 ° C, what is the volume at 100 ° C assuming constant pressure?
A) 0.40 L
B) 5.0 L
C) 2.5 L
D) 1.2 L
E) none of these
A) 0.40 L
B) 5.0 L
C) 2.5 L
D) 1.2 L
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
34
What pressure would a gas exert if a sample at 2.4 atm in a 2.0 L container is transferred to a 5.0 L container?
A) 0.96 atm
B) 2.4 atm
C) 6.0 atm
D) 1.8 atm
E) none of these
A) 0.96 atm
B) 2.4 atm
C) 6.0 atm
D) 1.8 atm
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
35
A 550. mL sample of gas at 2.00 torr of pressure is compressed to a volume oF4.00 mL. What is the pressure on the compressed sample?
A) 2.09×105 atm
B) 2.75 atm
C) 0.362 atm
D) 4.78×10 - 5 atm
E) none of these
A) 2.09×105 atm
B) 2.75 atm
C) 0.362 atm
D) 4.78×10 - 5 atm
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
36
A sample of gas is at 10 ° C and a pressure of 200 torr in a 2.40 L container. What is the pressure if the sample is moved to a 4.30 L container which is at 27 ° C?
A) 118 torr
B) 189 torr
C) 105 torr
D) 390 torr
E) none of these
A) 118 torr
B) 189 torr
C) 105 torr
D) 390 torr
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
37
A sample of gas at 27 ° C, 4.8 atm in a 2.0 L container is moved to a 1.0 L container at 0 ° C. What is the pressure?
A) 0.36 atm
B) 2.6 atm
C) 10.4 atm
D) 8.7 atm
E) none of these
A) 0.36 atm
B) 2.6 atm
C) 10.4 atm
D) 8.7 atm
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
38
A fixed quantity of gas at 23.0 ° C exhibits a pressure of 748 torr and occupies a volume of 10.3 L. What volume will this gas occupy at 165 ° C and constant pressure?
A) 1.44 L
B) 6.96 L
C) 15.2 L
D) 72.7 L
E) 73.9 L
A) 1.44 L
B) 6.96 L
C) 15.2 L
D) 72.7 L
E) 73.9 L

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
39
A gas occupies 3.8 L at 0.70 atm pressure. If this gas is allowed to expand tO6.5 L at constant temperature, what is the final pressure?
A) 5.8×10 - 2 atm
B) 0.41 atm
C) 1.2 atm
D) 17 atm
E) 35 atm
A) 5.8×10 - 2 atm
B) 0.41 atm
C) 1.2 atm
D) 17 atm
E) 35 atm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
40
A sample of NH3 gas that occupies a volume of 23.0 L at 10.0 ° C is heated at constant pressure until it fills a container with a volume of 50.0 L. What is the final temperature in ° C for this gas?
A) - 143 ° C
B) 21.7 ° C
C) 130 ° C
D) 342 ° C
E) 615 ° C
A) - 143 ° C
B) 21.7 ° C
C) 130 ° C
D) 342 ° C
E) 615 ° C

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
41
How would the Ideal Gas Law be rearranged to solve for the moles of gas ?
A) n = P×V×R×T
B)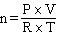
C)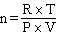
D)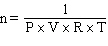
E)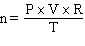
A) n = P×V×R×T
B)
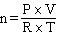
C)
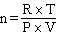
D)
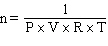
E)
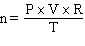

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
42
Carbon monoxide is a poisonous gas that is formed as a by-product of incomplete combustion. What pressure does 1.52 moles of this gas exert upon its surroundings if it is contained in a 42.5 L vessel at 65 ° C?
A) 0.191 atm
B) 0.430 atm
C) 0.992 atm
D) 2.33 atm
E) 1.79×103 atm
A) 0.191 atm
B) 0.430 atm
C) 0.992 atm
D) 2.33 atm
E) 1.79×103 atm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
43
Starting from the Combined Gas Law Equation given below, how is this equation rearranged to solve for T2 ?
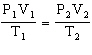 Combined Gas Law Equation
Combined Gas Law Equation
A)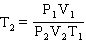
B)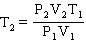
C)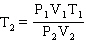
D)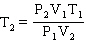
E)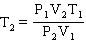
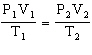 Combined Gas Law Equation
Combined Gas Law EquationA)
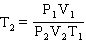
B)
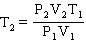
C)
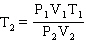
D)
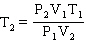
E)
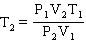

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
44
What are the units of the gas constant "R" when the pressure is given in atmospheres, the volume is given in liters, the amount of the gas is given in moles and the temperature is given in Kelvin?
A)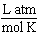
B)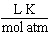
C)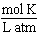
D)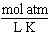
E)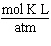
A)
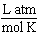
B)
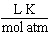
C)
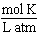
D)
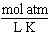
E)
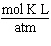

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
45
What is the volume in milliliters of 5.00 moles of helium gas held at a pressure of 1.50 atm and 27 ° C?
A) 7.40 mL
B) 12.0 ml
C) 7.39×103 mL
D) 8.21×104 mL
E) 1.85×105 mL
A) 7.40 mL
B) 12.0 ml
C) 7.39×103 mL
D) 8.21×104 mL
E) 1.85×105 mL

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
46
What is the pressure in atmospheres of 0.100 moles of O2 gas in a 2.0 Liter container at a temperature of 75 ° C?
A) 0.308 atm
B) 0.700 atm
C) 1.43 atm
D) 3.25 atm
E) 5.71 atm
A) 0.308 atm
B) 0.700 atm
C) 1.43 atm
D) 3.25 atm
E) 5.71 atm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
47
A helium weather balloon, when released, has a volume of 10.0 L at 27.0 ° C and a pressure of 663 mm Hg. What volume, in liters, will the balloon occupy at an altitude where the pressure is 96 mm Hg and the temperature is - 30.0 ° C?
A) - 76.7 L
B) 1.17 L
C) 1.79 L
D) 55.9 L
E) 85.3 L
A) - 76.7 L
B) 1.17 L
C) 1.79 L
D) 55.9 L
E) 85.3 L

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
48
What is the pressure in atmospheres for a 0.250 mole sample of CO2 gas that is held at 35 ° C in a 2.00 Liter container?
A) 0.316 atm
B) 0.359 atm
C) 3.16 atm
D) 5.75 atm
E) 12.6 atm
A) 0.316 atm
B) 0.359 atm
C) 3.16 atm
D) 5.75 atm
E) 12.6 atm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
49
A sample of carbon dioxide, CO2, gas has a volume of 15.2 L at a pressure of 1.35 atm and a temperature of 583 ° C. What is the temperature in degrees Celsius of this same sample of carbon dioxide gas at a pressure of 7.00 atm and a volume of 0.973 L?
A) - 117 ° C
B) 11.0 ° C
C) 99.4 ° C
D) 102 ° C
E) 922 ° C
A) - 117 ° C
B) 11.0 ° C
C) 99.4 ° C
D) 102 ° C
E) 922 ° C

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
50
What pressure will 4.2 g of O2 gas exert in a 4.0 L container at 27 ° C?
A) 0.60 atm
B) 0.81 atm
C) 1.2 atm
D) 2.4 atm
E) none of these
A) 0.60 atm
B) 0.81 atm
C) 1.2 atm
D) 2.4 atm
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
51
What is the volume of a container if 3.11 mol of a gas at 1.22 atm of pressure is at a temperature of 27 ° C?
A) 1.33 L
B) 62.8 L
C) 23.4 L
D) 84.2 L
E) none of these
A) 1.33 L
B) 62.8 L
C) 23.4 L
D) 84.2 L
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
52
What is the pressure inside a 3.2 L jar that contains 2.6 g of N2 gas at a temperature of 27 ° C?
A) 0.71 atm
B) 0.064 atm
C) 2.2 atm
D) 20 atm
E) none of these
A) 0.71 atm
B) 0.064 atm
C) 2.2 atm
D) 20 atm
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
53
What is the pressure of a container if 0.221 mol of a gas in a 1.22 L container is at a temperature of 27 ° C?
A) 4.11 atm
B) 3.22 atm
C) 4.46 atm
D) 0.212 atm
E) none of these
A) 4.11 atm
B) 3.22 atm
C) 4.46 atm
D) 0.212 atm
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
54
A fixed quantity of a gas at 25 ° C, occupies a volume of 5.00 L at 1.00 atm. What is the final temperature of this same gas if the pressure is increased to 2.00 atm and the volume is doubled to 10.0 L?
A) 75 ° C
B) 100 ° C
C) 919 ° C
D) 1192 ° C
E) 1465 ° C
A) 75 ° C
B) 100 ° C
C) 919 ° C
D) 1192 ° C
E) 1465 ° C

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
55
A sample of carbon dioxide gas has a volume of 15.2 L at a pressure of 1.35 atm and a temperature of 33 ° C. What is the temperature of this same gas in degrees celsius when the pressure is increased to 7.00 atm and the volume is decreased to 0.973 L?
A) - 171 ° C
B) 11.0 ° C
C) 101 ° C
D) 374 ° C
E) 649 ° C
A) - 171 ° C
B) 11.0 ° C
C) 101 ° C
D) 374 ° C
E) 649 ° C

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
56
A sample of carbon dioxide, CO2, gas has a volume of 364 mL at a pressure of 2.45 atm and a temperature of 100 ° C. What is the pressure of this same sample in atmospheres at a temperature of 225 ° C and a volume oF452 mL?
A) 0.80 atm
B) 0.877 atm
C) 1.48 atm
D) 2.63 atm
E) 4.44 atm
A) 0.80 atm
B) 0.877 atm
C) 1.48 atm
D) 2.63 atm
E) 4.44 atm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
57
What is the volume in liters of a container that holds 0.500 moles of O2 gas at 20.0 ° C, and 0.750 atm?
A) 6.24×10 - 2 L
B) 1.09 L
C) 9.02 L
D) 13.3 L
E) 16.0 L
A) 6.24×10 - 2 L
B) 1.09 L
C) 9.02 L
D) 13.3 L
E) 16.0 L

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
58
What is the volume in liters of a container that holds 0.100 moles of O2 gas at 10.0 ° C, and 2.00 atm?
A) 0.0411 L
B) 0.164 L
C) 1.16 L
D) 2.00 L
E) 4.65 L
A) 0.0411 L
B) 0.164 L
C) 1.16 L
D) 2.00 L
E) 4.65 L

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
59
What is the volume in liters of a sample of carbon monoxide at - 10 ° C and 389 torr if it occupies 3.65 L at 298 K and 770 torr?
A) - 55.0 L
B) 1.63 L
C) 2.09 L
D) 6.38 L
E) 8.19 L
A) - 55.0 L
B) 1.63 L
C) 2.09 L
D) 6.38 L
E) 8.19 L

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
60
A sample of CO gas has a volume of 7.31 L at a pressure of 735 mm of Hg and a temperature oF45 ° C. What would be the temperature in degrees Celsius of this same amount of gas at a pressure of 1275 mm of Hg and a volume of 0.800 L?
A) - 213 ° C
B) 8.5 ° C
C) 60.4 ° C
D) 333 ° C
E) 1402 ° C
A) - 213 ° C
B) 8.5 ° C
C) 60.4 ° C
D) 333 ° C
E) 1402 ° C

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
61
How many moles are present in a 2.00 L sample of gas at 30 ° C and 0.821 atm?
A) 18.5 mol
B) 1.50 mol
C) 0.667 mol
D) 6.60×10 - 2 mol
E) none of these
A) 18.5 mol
B) 1.50 mol
C) 0.667 mol
D) 6.60×10 - 2 mol
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
62
What is the molar mass of a gas if a 17.6 g sample exerts a pressure of 2.0 atm in a 4.0 L container at 27 ° C?
A) 54 g/mol
B) 42 g/mol
C) 66 g/mol
D) 28 g/mol
E) none of these
A) 54 g/mol
B) 42 g/mol
C) 66 g/mol
D) 28 g/mol
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
63
What is the molar mass of a gas at 374 torr and 45 ° C if 0.216 grams occupies a container that is 0.206 Liters?
A) 7.87 g/mol
B) 50.6 g/mol
C) 55.6 g/mol
D) 1.76×104 g/mol
E) 3.78×105 g/mol
A) 7.87 g/mol
B) 50.6 g/mol
C) 55.6 g/mol
D) 1.76×104 g/mol
E) 3.78×105 g/mol

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
64
A sample of chlorine gas is confined in a 6.20 liter container at 202 torr and 27 ° C. How many grams of chlorine gas are present in the sample?
Molar Mass(Cl2) = 70.90 g/mol
A) 0.0669 g Cl2
B) 0.743 g Cl2
C) 4.74 g Cl2
D) 50.8 g Cl2
E) 52.7 g Cl2
Molar Mass(Cl2) = 70.90 g/mol
A) 0.0669 g Cl2
B) 0.743 g Cl2
C) 4.74 g Cl2
D) 50.8 g Cl2
E) 52.7 g Cl2

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
65
What is the density of O2 gas at 24 ° C and 1.00 atm?
A) 1.31 g/L
B) 1.51 g/L
C) 1.81 g/L
D) 0.931 g/L
E) none of these
A) 1.31 g/L
B) 1.51 g/L
C) 1.81 g/L
D) 0.931 g/L
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
66
What is the molar mass of a gas if 5.0 g occupies 8.2 L at 0.500 atm and 27 ° C?
A) 40 g/mol
B) 30 g/mol
C) 15 g/mol
D) 45 g/mol
E) none of these
A) 40 g/mol
B) 30 g/mol
C) 15 g/mol
D) 45 g/mol
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
67
What is the density of a sample of O2 gas if it exerts a pressure of 1.38 atm at 300 K?
A) 8.20 g/L
B) 0.210 g/L
C) 2.42 g/L
D) 1.79 g/L
E) none of these
A) 8.20 g/L
B) 0.210 g/L
C) 2.42 g/L
D) 1.79 g/L
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
68
What is the molar mass of a gas if 10 g occupies 8.2 L at 380 torr and 27 ° C?
A) 90 g/mol
B) 30 g/mol
C) 60 g/mol
D) 80 g/mol
E) none of these
A) 90 g/mol
B) 30 g/mol
C) 60 g/mol
D) 80 g/mol
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
69
What is the temperature of a 1.00 L container if 0.0112 mol of a gas is at 721 torr of pressure?
A) 435 ° C
B) 982 ° C
C) 255 ° C
D) 759 ° C
E) none of these
A) 435 ° C
B) 982 ° C
C) 255 ° C
D) 759 ° C
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
70
What mass of N2 gas is in a 4.20 L container at a pressure of 1.10 atm at 27 ° C?
A) 58.4 g
B) 73.5 g
C) 5.25 g
D) 10.2 g
E) none of these
A) 58.4 g
B) 73.5 g
C) 5.25 g
D) 10.2 g
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
71
How many moles of chlorine, Cl2, gas is present if this gas is contained in a 9.22 L vessel at 1124 torr and 24 ° C?
A) 0.145 moles Cl2
B) 0.559 moles Cl2
C) 1.79 moles Cl2
D) 6.92 moles Cl2
E) 425 mole Cl2
A) 0.145 moles Cl2
B) 0.559 moles Cl2
C) 1.79 moles Cl2
D) 6.92 moles Cl2
E) 425 mole Cl2

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
72
Which gas would be the densest at 1.00 atm and 298 K?
A) CO2
B) N2O
C) Cl2
D) All of these gases have an identical density at 298 K
E) There is not enough information to determine which gas is most dense.
A) CO2
B) N2O
C) Cl2
D) All of these gases have an identical density at 298 K
E) There is not enough information to determine which gas is most dense.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
73
What is the density of SO2 gas at 211 ° C and 1.00 atm?
A) 1.33 g/L
B) 1.61 g/L
C) 1.88 g/L
D) 0.921 g/L
E) none of these
A) 1.33 g/L
B) 1.61 g/L
C) 1.88 g/L
D) 0.921 g/L
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
74
What is the molar mass of a gas if 16 g occupy 3.0 L at 2.2 atm and 27 ° C?
A) 30 g/mol
B) 45 g/mol
C) 60 g/mol
D) 90 g/mol
E) none of these
A) 30 g/mol
B) 45 g/mol
C) 60 g/mol
D) 90 g/mol
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
75
What is the density of SF6 gas at 105 ° C and 1.6 atm?
MM(SF6) = 146.07 g/mol
A) 0.18 g/L
B) 0.34 g/L
C) 7.5 g/L
D) 27 g/L
E) 50 g/L
MM(SF6) = 146.07 g/mol
A) 0.18 g/L
B) 0.34 g/L
C) 7.5 g/L
D) 27 g/L
E) 50 g/L

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
76
How many moles of neon gas are present in a 10.0 L vessel, if the pressure of this gas equals 1.05 atm at 30 ° C?
A) 0.235 moles Ne
B) 0.422 moles Ne
C) 2.37 moles Ne
D) 4.26 moles Ne
E) 3.88×104 moles Ne
A) 0.235 moles Ne
B) 0.422 moles Ne
C) 2.37 moles Ne
D) 4.26 moles Ne
E) 3.88×104 moles Ne

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
77
How many moles of a gas occupy a volume of 5.0 L and exhibit a pressure of 775 torr at 25 ° C?
A) 0.21 moles
B) 2.5 moles
C) 4.8 moles
D) 160 moles
E) 1900 moles
A) 0.21 moles
B) 2.5 moles
C) 4.8 moles
D) 160 moles
E) 1900 moles

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
78
What is the density of Freon - 11 (CFCl3) gas at 105 ° C and 1.6 atm?
Molar Mass(CFCl3) = 137.36 g/mol
A) 0.36 g/L
B) 7.1 g/L
C) 26 g/L
D) 2.8×105 g/L
E) 4.0×105 g/L
Molar Mass(CFCl3) = 137.36 g/mol
A) 0.36 g/L
B) 7.1 g/L
C) 26 g/L
D) 2.8×105 g/L
E) 4.0×105 g/L

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
79
How many grams of O2 would be in a 1.0 L container at a pressure of 600 torr and temperature of 27 ° C?
A) 1.0 g
B) 4.2 g
C) 24.3 g
D) 0.36 g
E) 0.32 g
A) 1.0 g
B) 4.2 g
C) 24.3 g
D) 0.36 g
E) 0.32 g

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
80
What is the molar mass of a gas if 2.50 g occupies 0.875 L at 685 torr and 35 ° C?
A) 9.50 g/mol
B) 80.2 g/mol
C) 83.4 g/mol
D) 88.4 g/mol
E) None of these
A) 9.50 g/mol
B) 80.2 g/mol
C) 83.4 g/mol
D) 88.4 g/mol
E) None of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck


