Deck 10: Molecular Structure and Bonding Theories
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/133
Play
Full screen (f)
Deck 10: Molecular Structure and Bonding Theories
1
What bonded atom lone pair arrangement is predicted by VSEPR theory for the electron groups that surround the carbon atom in CO2?
A) Linear
B) Trigonal planar
C) Bent
D) Tetrahedral
E) Trigonal pyramidal
A) Linear
B) Trigonal planar
C) Bent
D) Tetrahedral
E) Trigonal pyramidal
Linear
2
What is the steric number with respect to the central iodine atom in the Lewis structure for the species, IF41 - ?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6
6
3
What is the bonded atom lone pair arrangement with respect to the electrons around the central bromine atom in the ion BrO31 - ?
A) Linear
B) Trigonal planar
C) Tetrahedral
D) Trigonal bipyramidal
E) Octahedral
A) Linear
B) Trigonal planar
C) Tetrahedral
D) Trigonal bipyramidal
E) Octahedral
Tetrahedral
4
What is the steric number with respect to the central nitrogen atom in the Lewis structure for ammonia, NH3?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
5
What is the steric number with respect to the central oxygen atom in the Lewis structure for ozone, O3?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
6
What bonded atom lone pair arrangement is predicted by VSEPR theory for the electron groups that surround the nitrogen atom in NH3?
A) Linear
B) Trigonal planar
C) Bent
D) Tetrahedral
E) Trigonal pyramidal
A) Linear
B) Trigonal planar
C) Bent
D) Tetrahedral
E) Trigonal pyramidal

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
7
What is the steric number with respect to the central iodine atom in the Lewis structure for the species, ICl21 - ?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
8
What is the steric number with respect to the central sulfur atom in the Lewis structure for sulfur dioxide, SO2?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
9
What bonded atom lone pair arrangement is predicted by VSEPR theory for the electron groups that surround the chlorine atom in ClF3?
A) Linear
B) Trigonal planar
C) V-shaped (bent)
D) Tetrahedral
E) Trigonal bipyramidal
A) Linear
B) Trigonal planar
C) V-shaped (bent)
D) Tetrahedral
E) Trigonal bipyramidal

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
10
What is the bonded atom lone pair arrangement with respect to the electrons around the central oxygen atom in O3?
A) Linear
B) Trigonal planar
C) Trigonal pyramidal
D) Tetrahedral
E) Bent
A) Linear
B) Trigonal planar
C) Trigonal pyramidal
D) Tetrahedral
E) Bent

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
11
The bonded atom lone pair arrangement about the carbon atoms in H2CCH2 is:
A) trigonal planar.
B) tetrahedral.
C) trigonal bipyramidal.
D) octahedral.
E) none of these
A) trigonal planar.
B) tetrahedral.
C) trigonal bipyramidal.
D) octahedral.
E) none of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
12
What is the bonded atom lone pair arrangement with respect to the electrons around the sulfur atom in SO2?
A) Linear
B) Trigonal planar
C) Trigonal pyramidal
D) Tetrahedral
E) Bent
A) Linear
B) Trigonal planar
C) Trigonal pyramidal
D) Tetrahedral
E) Bent

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
13
The bonded atom lone pair arrangement about the nitrogen atoms in HNNH is:
A) trigonal planar.
B) tetrahedral.
C) trigonal bipyramidal.
D) octahedral.
E) none of these
A) trigonal planar.
B) tetrahedral.
C) trigonal bipyramidal.
D) octahedral.
E) none of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
14
What is the steric number with respect to the central carbon atom in the Lewis structure for carbon dioxide, CO2?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
15
What is the bonded atom lone pair arrangement with respect to the electrons around the central iodine atom in the species, IF41 - ?
A) Tetrahedral
B) Trigonal bipyramidal
C) See-Saw
D) Octahedral
E) Square base pyramid
A) Tetrahedral
B) Trigonal bipyramidal
C) See-Saw
D) Octahedral
E) Square base pyramid

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
16
What is the steric number with respect to the central chlorine atom in the Lewis structure for chlorine trifluoride, ClF3?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
17
What bonded atom lone pair arrangement would be assumed by the electron groups around the central phosphorous atom in phosphite, PO33 - ?
A) Linear
B) Angular
C) Trigonal planar
D) Trigonal pyramidal
E) Tetrahedral
A) Linear
B) Angular
C) Trigonal planar
D) Trigonal pyramidal
E) Tetrahedral

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
18
What is the bonded atom lone pair arrangement with respect to the iodine atom in the species ICl21 - ?
A) Linear
B) Trigonal planar
C) V-shaped (bent)
D) Tetrahedral
E) Trigonal bipyramidal
A) Linear
B) Trigonal planar
C) V-shaped (bent)
D) Tetrahedral
E) Trigonal bipyramidal

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
19
The bonded atom lone pair arrangement about the nitrogen atom in NF3 is:
A) trigonal planar.
B) tetrahedral.
C) trigonal bipyramidal.
D) octahedral.
E) none of these
A) trigonal planar.
B) tetrahedral.
C) trigonal bipyramidal.
D) octahedral.
E) none of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
20
The bonded atom lone pair arrangement about the sulfur atom in SF4 is:
A) trigonal planar.
B) tetrahedral.
C) trigonal bipyramidal.
D) octahedral.
E) none of these
A) trigonal planar.
B) tetrahedral.
C) trigonal bipyramidal.
D) octahedral.
E) none of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following molecules would have a Lewis structure that most closely resembles SiO2, as well as having an identical bonded atom lone pair arrangement and an identical molecular shape?
A) H2O
B) C S2
C) SCl2
D) SO2
E) XeF2
A) H2O
B) C S2
C) SCl2
D) SO2
E) XeF2

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
22
What molecule listed below has a square planar molecular shape?
A) XeF4
B) SF4
C) SiF4
D) CF4
E) ClF5
A) XeF4
B) SF4
C) SiF4
D) CF4
E) ClF5

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
23
What is the molecular shape of NI3?
A) Trigonal plane
B) Linear
C) Tetrahedral
D) Trigonal pyramid
E) None of these
A) Trigonal plane
B) Linear
C) Tetrahedral
D) Trigonal pyramid
E) None of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following molecules would have a Lewis structure that most closely resembles CCl4, as well as having an identical bonded atom lone pair arrangement and an identical molecular shape?
A) SF4
B) XeF4
C) SiBr4
D) IF41 -
E) None of these
A) SF4
B) XeF4
C) SiBr4
D) IF41 -
E) None of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
25
What molecular shape would you predict for the bonding around Ge in GeCl4?
A) Square planar
B) Trigonal
C) Tetrahedral
D) Trigonal pyramid
E) None of these
A) Square planar
B) Trigonal
C) Tetrahedral
D) Trigonal pyramid
E) None of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following molecules has a central atom that has a trigonal planar bonded atom lone pair arrangement?
A) SO3
B) IF3
C) NF3
D) All of these
E) None of these
A) SO3
B) IF3
C) NF3
D) All of these
E) None of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
27
What molecular shape is assumed by the four atoms in the molecule, ClF3?
A) Trigonal pyramidal
B) Trigonal planar
C) T-shaped
D) Tetrahedral
E) Trigonal bipyramidal
A) Trigonal pyramidal
B) Trigonal planar
C) T-shaped
D) Tetrahedral
E) Trigonal bipyramidal

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
28
What molecular shape is assumed by the three atoms in the ion, ICl21 - ?
A) Linear
B) Trigonal planar
C) Bent
D) Tetrahedral
E) Trigonal bipyramidal
A) Linear
B) Trigonal planar
C) Bent
D) Tetrahedral
E) Trigonal bipyramidal

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
29
Three statements are given below. Pick the best answer.
I. BCl3 is a trigonal planar molecule.
II. SF6 is an octahedral molecule.
III. PH3 is a trigonal planar molecule.
A) I and II are correct, III is not
B) I and III are correct, II is not
C) II and III are correct, I is not
D) all are true
E) only I is true
I. BCl3 is a trigonal planar molecule.
II. SF6 is an octahedral molecule.
III. PH3 is a trigonal planar molecule.
A) I and II are correct, III is not
B) I and III are correct, II is not
C) II and III are correct, I is not
D) all are true
E) only I is true

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
30
What molecule listed below has a see-saw molecular shape?
A) XeF4
B) SF4
C) SiF4
D) CF4
E) ClF5
A) XeF4
B) SF4
C) SiF4
D) CF4
E) ClF5

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
31
What molecular shape is assumed by the four atoms in the phosphite anion, PO33 - ?
A) Linear
B) Angular
C) Trigonal planar
D) Trigonal pyramidal
E) Tetrahedral
A) Linear
B) Angular
C) Trigonal planar
D) Trigonal pyramidal
E) Tetrahedral

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
32
What molecular shape is assumed by the five atoms in the ion, IF41 - ?
A) Tetrahedral
B) Square planar
C) See-Saw
D) Octahedral
E) Square base pyramid
A) Tetrahedral
B) Square planar
C) See-Saw
D) Octahedral
E) Square base pyramid

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
33
What molecular shape is assumed by the three atoms in the molecule, SO2?
A) Linear
B) Trigonal planar
C) Trigonal pyramidal
D) Tetrahedral
E) Bent
A) Linear
B) Trigonal planar
C) Trigonal pyramidal
D) Tetrahedral
E) Bent

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
34
What is the bonded atom lone pair arrangement with respect to the electron groups around the central nitrogen atom in the nitrate anion, NO31 - ?
A) Angular
B) Linear
C) Trigonal planar
D) Trigonal pyramidal
E) T-shape
A) Angular
B) Linear
C) Trigonal planar
D) Trigonal pyramidal
E) T-shape

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following molecules would have a Lewis structure that most closely resembles BBr3, as well as having an identical bonded atom lone pair arrangement and an identical molecular shape?
A) NCl3
B) ClF3
C) AlCl3
D) PCl3
E) None of these
A) NCl3
B) ClF3
C) AlCl3
D) PCl3
E) None of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
36
What molecular shape is assumed by the four atoms in the molecule, NH3?
A) Linear
B) Trigonal planar
C) Bent
D) Tetrahedral
E) Trigonal pyramidal
A) Linear
B) Trigonal planar
C) Bent
D) Tetrahedral
E) Trigonal pyramidal

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
37
What molecular shape is assumed by the four atoms in the nitrate anion, NO31 - ?
A) Angular
B) Linear
C) Trigonal planar
D) Trigonal pyramidal
E) T-shape
A) Angular
B) Linear
C) Trigonal planar
D) Trigonal pyramidal
E) T-shape

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following molecules has a central atom that has a tetrahedral bonded atom lone pair arrangement?
A) CCl4
B) NF3
C) H2S
D) All of these
E) None of these
A) CCl4
B) NF3
C) H2S
D) All of these
E) None of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
39
What molecular shape is assumed by the three atoms in the molecule, CO2?
A) Linear
B) Trigonal planar
C) Bent
D) Tetrahedral
E) Trigonal pyramidal
A) Linear
B) Trigonal planar
C) Bent
D) Tetrahedral
E) Trigonal pyramidal

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following molecules would have a Lewis structure that most closely resembles O3, as well as having an identical bonded atom lone pair arrangement and an identical molecular shape?
A) H2S
B) CO2
C) OCl2
D) SO2
E) KrF2
A) H2S
B) CO2
C) OCl2
D) SO2
E) KrF2

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
41
Given the three statements below, pick the best answer.
I. [AlCl4] - is tetrahedral.
II. The Cl - N - Cl angle in NCl3 is about 120 ° .
III. The H - S - H angle in H2S is about 109 ° .
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) all three are true
E) only I is true
I. [AlCl4] - is tetrahedral.
II. The Cl - N - Cl angle in NCl3 is about 120 ° .
III. The H - S - H angle in H2S is about 109 ° .
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) all three are true
E) only I is true

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
42
What is the H - C - H bond angle in CH4?
A) 90 °
B) 109 °
C) 120 °
D) 180 °
E) a and c
A) 90 °
B) 109 °
C) 120 °
D) 180 °
E) a and c

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
43
Exhibit 10-1 Consider the molecule IF 3 to answer the following question(s). In theory, there are three possible molecular shapes for the molecule IF 3 as shown below. I. 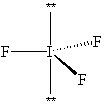
II.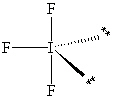
III.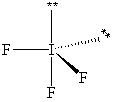
Refer to Exhibit 10-1. After assessing significant interactions (LP - LP, LP - BP and BP - BP), arrange the three shapes in order of least favorable to most favorable molecular shape.
A) (least favorable) I < II < III (most favorable)
B) (least favorable) I < III < II (most favorable)
C) (least favorable) II < I < III (most favorable)
D) (least favorable) II < III < I (most favorable)
E) (least favorable) III < I < II (most favorable)

II.

III.

Refer to Exhibit 10-1. After assessing significant interactions (LP - LP, LP - BP and BP - BP), arrange the three shapes in order of least favorable to most favorable molecular shape.
A) (least favorable) I < II < III (most favorable)
B) (least favorable) I < III < II (most favorable)
C) (least favorable) II < I < III (most favorable)
D) (least favorable) II < III < I (most favorable)
E) (least favorable) III < I < II (most favorable)

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
44
The H - X - H angle is largest in which species below?
A) H2O
B) NH3
C) H2CO
D) BeH2
E) CH4
A) H2O
B) NH3
C) H2CO
D) BeH2
E) CH4

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
45
In the reaction BH3 + NH3→H3B - NH3 the H - B - H bond angles change from about:
A) 109 ° to 120 °
B) 90 ° to 109 °
C) 120 ° to 109 °
D) are 109 ° in both reactant and product
E) are 120 ° in both reactant and product
A) 109 ° to 120 °
B) 90 ° to 109 °
C) 120 ° to 109 °
D) are 109 ° in both reactant and product
E) are 120 ° in both reactant and product

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
46
Below is a structural formula for methyl formate. Complete its Lewis structure. What are the approximate bond angles (A-C) in the figure below for methyl formate?
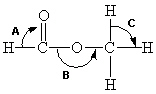
A) A = 90°, B = 180°, C = 90°
B) A = 120°, B = 180°, C = 109°
C) A = 120°, B = 109°, C = 90°
D) A = 109°, B = 109°, C = 109°
E) A = 120°, B = 109°, C = 109°

A) A = 90°, B = 180°, C = 90°
B) A = 120°, B = 180°, C = 109°
C) A = 120°, B = 109°, C = 90°
D) A = 109°, B = 109°, C = 109°
E) A = 120°, B = 109°, C = 109°

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
47
Consider the molecule shown below of carbonic acid. Complete its Lewis structure. What are the two approximate bond angles represented by A and B in the figure below?
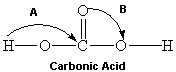
A) A = 180° and B = 90°
B) A = 109° and B = 90°
C) A = 180° and B = 109°
D) A = 109° and B = 120°
E) A = 180° and B = 120°

A) A = 180° and B = 90°
B) A = 109° and B = 90°
C) A = 180° and B = 109°
D) A = 109° and B = 120°
E) A = 180° and B = 120°

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
48
What value listed below best approximates the H - O - H bond angle in water?
A) 90 °
B) 104.5 °
C) 109 °
D) 120 °
E) 180 °
A) 90 °
B) 104.5 °
C) 109 °
D) 120 °
E) 180 °

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
49
Which molecule listed below has the largest F - X - F angle (X = central atoms)?
A) OF2
B) NF3
C) CF4
D) PF3
E) XeF2
A) OF2
B) NF3
C) CF4
D) PF3
E) XeF2

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
50
Below is a structural formula of ethanol. Complete its Lewis structure. What are the approximate values for the two bond angles indicated by A and B in the figure?
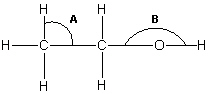
A) A = 90° and B = 180°
B) A = 109° and B = 180°
C) A = 90° and B = 120°
D) A = 109° and B = 120°
E) A = 109° and B = 109°

A) A = 90° and B = 180°
B) A = 109° and B = 180°
C) A = 90° and B = 120°
D) A = 109° and B = 120°
E) A = 109° and B = 109°

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
51
What is the approximate Cl - C - Cl angle in Cl2CO (note the 2 Cl atoms and the O atom are bound to the C and not to each other)?
A) 90 °
B) 109 °
C) 120 °
D) 180 °
E) none of these
A) 90 °
B) 109 °
C) 120 °
D) 180 °
E) none of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
52
Exhibit 10-1 Consider the molecule IF 3 to answer the following question(s). In theory, there are three possible molecular shapes for the molecule IF 3 as shown below.
I.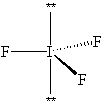
II.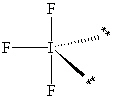
III.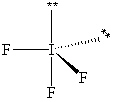
Refer to Exhibit 10-1. How many significant (90 or less) lone pair/lone pair (LP - LP) interactions are present in each shape?
A) Shape I = 0; Shape II = 0; Shape III = 0
B) Shape I = 0; Shape II = 0; Shape III = 1
C) Shape I = 0; Shape II = 1; Shape III = 0
D) Shape I = 1; Shape II = 0; Shape III = 0
E) Shape I = 1; Shape II = 1; Shape III = 1
I.

II.

III.

Refer to Exhibit 10-1. How many significant (90 or less) lone pair/lone pair (LP - LP) interactions are present in each shape?
A) Shape I = 0; Shape II = 0; Shape III = 0
B) Shape I = 0; Shape II = 0; Shape III = 1
C) Shape I = 0; Shape II = 1; Shape III = 0
D) Shape I = 1; Shape II = 0; Shape III = 0
E) Shape I = 1; Shape II = 1; Shape III = 1

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
53
Below is a structural formula of acetic acid. Complete its Lewis structure. What are the approximate bond angles as predicted by VSEPR theory for angles A-C?
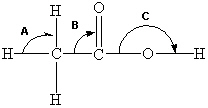
A) A = 90°, B = 90°, C = 180°
B) A = 109°, B = 90°, C = 180°
C) A = 90°, B = 109°, C = 180°
D) A = 109°, B = 120°, C = 109°
E) A = 109°, B = 90°, C = 109°

A) A = 90°, B = 90°, C = 180°
B) A = 109°, B = 90°, C = 180°
C) A = 90°, B = 109°, C = 180°
D) A = 109°, B = 120°, C = 109°
E) A = 109°, B = 90°, C = 109°

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
54
What is the O - S - O bond angle in SO3?
A) 90 °
B) 109 °
C) 120 °
D) 180 °
E) 90 ° and 120 °
A) 90 °
B) 109 °
C) 120 °
D) 180 °
E) 90 ° and 120 °

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
55
What is the approximate H - N - H bond angle in a molecule of NH3?
A) 90 °
B) 107 °
C) 120 °
D) 180 °
E) None of these
A) 90 °
B) 107 °
C) 120 °
D) 180 °
E) None of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
56
Pick the one statement listed below that is true .
A) The H - S - H angle in H2S is about 180 ° .
B) The Cl - P - Cl angles in PCl3 are all about 120 ° .
C) The F - Al - F angles in AlF3 are all about 120 ° .
D) The O - S - O angle in SO2 is 180 ° .
E) All 4 answers are false.
A) The H - S - H angle in H2S is about 180 ° .
B) The Cl - P - Cl angles in PCl3 are all about 120 ° .
C) The F - Al - F angles in AlF3 are all about 120 ° .
D) The O - S - O angle in SO2 is 180 ° .
E) All 4 answers are false.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
57
Given the three statements below, pick the best answer.
I. The H - N - H angle in NH3 should be about 109 ° .
II. SF4 is tetrahedral.
III. SO2 is linear.
A) I and II are true, III is not
B) I and III are true, II is not
C) II and III are true, I is not
D) all three are true
E) only I is true
I. The H - N - H angle in NH3 should be about 109 ° .
II. SF4 is tetrahedral.
III. SO2 is linear.
A) I and II are true, III is not
B) I and III are true, II is not
C) II and III are true, I is not
D) all three are true
E) only I is true

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
58
The H - C - H angle in CH2Cl2 is approximately:
A) 109 °
B) 120 °
C) 180 °
D) 90 °
E) none of these
A) 109 °
B) 120 °
C) 180 °
D) 90 °
E) none of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
59
Based upon VSEPR theory, what are the two approximate bond angles labeled A and B respectively in the following molecule of ethanal?
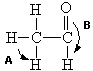
A) A = 90°, B = 180°
B) A = 109°, B = 180°
C) A = 109°, B = 120°
D) A = 90°, B = 120°
E) A = 109°, B = 109°

A) A = 90°, B = 180°
B) A = 109°, B = 180°
C) A = 109°, B = 120°
D) A = 90°, B = 120°
E) A = 109°, B = 109°

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
60
The H - C - H angle in H3CCl is approximately:
A) 90 °
B) 180 °
C) 120 °
D) 109 °
E) none of these
A) 90 °
B) 180 °
C) 120 °
D) 109 °
E) none of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following molecules is(are) non-polar ?
I. O3 (angular)
II. H2O (angular)
III. CO2 (linear)
A) I only
B) I and II
C) I and III
D) II and III
E) All of these
I. O3 (angular)
II. H2O (angular)
III. CO2 (linear)
A) I only
B) I and II
C) I and III
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
62
Which molecule from the list below is considered polar ?
The molecular shape is listed in parenthesis.
A) NCl3 (trigonal pyramidal)
B) CO2 (linear)
C) CH4 (tetrahedral)
D) N2 (linear)
E) SO3 (trigonal planar)
The molecular shape is listed in parenthesis.
A) NCl3 (trigonal pyramidal)
B) CO2 (linear)
C) CH4 (tetrahedral)
D) N2 (linear)
E) SO3 (trigonal planar)

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
63
From the list of compounds provided below, which compound is polar ?
A) CCl4
B) C S2
C) SO3
D) SF4
E) PF5
A) CCl4
B) C S2
C) SO3
D) SF4
E) PF5

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
64
Given the three statements below, pick the best answer.
I. HBF2 is a non-polar molecule.
II. CF4 is a non-polar molecule.
III. NF3 is a non-polar molecule.
A) only I is correct
B) only II is correct
C) only III is correct
D) II and III are correct, I is not
E) all three are incorrect
I. HBF2 is a non-polar molecule.
II. CF4 is a non-polar molecule.
III. NF3 is a non-polar molecule.
A) only I is correct
B) only II is correct
C) only III is correct
D) II and III are correct, I is not
E) all three are incorrect

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
65
Which statement below best describes CO2 with respect to the polarity of bonds and the polarity of the molecule?
A) CO2 has polar C - O bonds and is a polar molecule .
B) CO2 has polar C - O bonds and is a non-polar molecule .
C) CO2 has non-polar C - O bonds and is a polar molecule .
D) CO2 has non-polar C - O bonds and is a non-polar molecule .
E) CO2 is neither a polar nor a non-polar molecule but is rather an Ionic compound.
A) CO2 has polar C - O bonds and is a polar molecule .
B) CO2 has polar C - O bonds and is a non-polar molecule .
C) CO2 has non-polar C - O bonds and is a polar molecule .
D) CO2 has non-polar C - O bonds and is a non-polar molecule .
E) CO2 is neither a polar nor a non-polar molecule but is rather an Ionic compound.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following molecules is(are) polar?
I. SO2 (angular)
II. OF2 (angular)
III. C S2 (linear)
A) I only
B) II only
C) III only
D) I and II
E) II and III
I. SO2 (angular)
II. OF2 (angular)
III. C S2 (linear)
A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the molecules listed below is polar?
A) SiCl4
B) PCl5
C) AsCl3
D) AlCl3
E) all are non-polar
A) SiCl4
B) PCl5
C) AsCl3
D) AlCl3
E) all are non-polar

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following molecule is considered nonpolar ?
The molecular geometry is listed in parenthesis.
A) PCl3 (trigonal pyramidal)
B) CHCl3 (tetrahedral)
C) CO2 (linear)
D) SO2 (angular)
E) HCN (linear)
The molecular geometry is listed in parenthesis.
A) PCl3 (trigonal pyramidal)
B) CHCl3 (tetrahedral)
C) CO2 (linear)
D) SO2 (angular)
E) HCN (linear)

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
69
Which statement below best describes SO2 with respect to the polarity of bonds and the polarity of the molecule?
A) SO2 has polar S - O bonds and is a polar molecule .
B) SO2 has polar S - O bonds and is a non-polar molecule .
C) SO2 has non-polar S - O bonds and is a polar molecule .
D) SO2 has non-polar S - O bonds and is a non-polar molecule .
E) SO2 is neither a polar nor a non-polar molecule but is rather an Ionic compound.
A) SO2 has polar S - O bonds and is a polar molecule .
B) SO2 has polar S - O bonds and is a non-polar molecule .
C) SO2 has non-polar S - O bonds and is a polar molecule .
D) SO2 has non-polar S - O bonds and is a non-polar molecule .
E) SO2 is neither a polar nor a non-polar molecule but is rather an Ionic compound.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following molecules is(are) polar ?
I. N2
II. HCl
III. Cl2
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. N2
II. HCl
III. Cl2
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
71
Pick the one statement below that is true.
A) CO2 is polar.
B) H2S is non-polar.
C) HCCl3 is non-polar.
D) PF5 is polar.
E) All of these statements are false.
A) CO2 is polar.
B) H2S is non-polar.
C) HCCl3 is non-polar.
D) PF5 is polar.
E) All of these statements are false.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following molecules is(are) polar ?
I. NH3 (trigonal pyramidal)
II. SO3 (trigonal planar)
III. ClF3 (T-shaped)
A) II only
B) I and II
C) I and III
D) II and III
E) All of these
I. NH3 (trigonal pyramidal)
II. SO3 (trigonal planar)
III. ClF3 (T-shaped)
A) II only
B) I and II
C) I and III
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following molecules is(are) polar ?
I. BF3 (trigonal planar)
II. NH3 (trigonal pyramidal)
III. BF2Cl (trigonal planar)
A) I only
B) I and II
C) I and III
D) II and III
E) All of these
I. BF3 (trigonal planar)
II. NH3 (trigonal pyramidal)
III. BF2Cl (trigonal planar)
A) I only
B) I and II
C) I and III
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following molecules is(are) nonpolar ?
I. SiCl4 (tetrahedral)
II. CHCl3 (tetrahedral)
III. CH2Cl2 (tetrahedral)
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. SiCl4 (tetrahedral)
II. CHCl3 (tetrahedral)
III. CH2Cl2 (tetrahedral)
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
75
Given the three molecules listed below, which choice is correct?
I. H2S
II. BF3
III. PF3
A) all are polar
B) I and II are polar, III is non-polar
C) I and III are polar, II is non-polar
D) III is polar, I and II are non-polar
E) none of these choices are correct
I. H2S
II. BF3
III. PF3
A) all are polar
B) I and II are polar, III is non-polar
C) I and III are polar, II is non-polar
D) III is polar, I and II are non-polar
E) none of these choices are correct

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
76
Which statement below best describes NH3 with respect to the polarity of bonds and the polarity of the molecule?
A) NH3 has polar N - H bonds and is a polar molecule .
B) NH3 has polar N - H bonds and is a non-polar molecule .
C) NH3 has non-polar N - H bonds and is a polar molecule .
D) NH3 has non-polar N - H bonds and is a non-polar molecule .
E) NH3 is neither a polar nor a non-polar molecule but is rather an Ionic compound.
A) NH3 has polar N - H bonds and is a polar molecule .
B) NH3 has polar N - H bonds and is a non-polar molecule .
C) NH3 has non-polar N - H bonds and is a polar molecule .
D) NH3 has non-polar N - H bonds and is a non-polar molecule .
E) NH3 is neither a polar nor a non-polar molecule but is rather an Ionic compound.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following molecules is non-polar?
A) BCl3
B) H2O
C) NCl3
D) PCl3
E) all are polar
A) BCl3
B) H2O
C) NCl3
D) PCl3
E) all are polar

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following is a non-polar molecule that contains polar bonds?
A) H2O
B) SO2
C) CCl4
D) Cl2
E) none of these
A) H2O
B) SO2
C) CCl4
D) Cl2
E) none of these

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the molecules listed below is polar?
A) BH3
B) PH3
C) CF4
D) PF5
E) all are non-polar
A) BH3
B) PH3
C) CF4
D) PF5
E) all are non-polar

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following molecules is(are) polar ?
I. NF3 (trigonal pyramidal)
II. BF3 (trigonal planar)
III. IF3 (T-shape)
A) I only
B) II only
C) III only
D) I and II
E) I and III
I. NF3 (trigonal pyramidal)
II. BF3 (trigonal planar)
III. IF3 (T-shape)
A) I only
B) II only
C) III only
D) I and II
E) I and III

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck


