Deck 15: Solutions of Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/179
Play
Full screen (f)
Deck 15: Solutions of Acids and Bases
1
Which of the following is a property of an acid?
I. Slippery to touch.
II. Tastes sour.
III. Reacts with active metals to form H2 gas.
A) I only
B) II only
C) I and II
D) II and III
E) All of these
I. Slippery to touch.
II. Tastes sour.
III. Reacts with active metals to form H2 gas.
A) I only
B) II only
C) I and II
D) II and III
E) All of these
II and III
2
What is the conjugate acid of the species H2BO31 - ?
A) H3BO3
B) H3BO32 -
C) H3O+
D) HBO32 -
E) HBO3
A) H3BO3
B) H3BO32 -
C) H3O+
D) HBO32 -
E) HBO3
H3BO3
3
For the reaction CN - (aq) + H₂ O ( 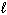 )
) 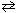 HCN (aq) + OH - (aq), one of the acid-base conjugate pairs is:
HCN (aq) + OH - (aq), one of the acid-base conjugate pairs is:
A) HCN, H2O
B) HCN, CN -
C) H2O, CN -
D) HCN, OH -
E) CN - , OH -
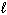 )
) 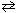 HCN (aq) + OH - (aq), one of the acid-base conjugate pairs is:
HCN (aq) + OH - (aq), one of the acid-base conjugate pairs is:A) HCN, H2O
B) HCN, CN -
C) H2O, CN -
D) HCN, OH -
E) CN - , OH -
HCN, CN -
4
Which of the following is a property of a base?
I. Slippery to touch.
II. Tastes bitter.
III. Reacts with active metals to form H2 gas.
A) I only
B) II only
C) I and II
D) II and III
E) All of these
I. Slippery to touch.
II. Tastes bitter.
III. Reacts with active metals to form H2 gas.
A) I only
B) II only
C) I and II
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the following Bronsted-Lowery acid-base reaction:
HClO ₂ + N(CH ₃) ₃![<strong>Consider the following Bronsted-Lowery acid-base reaction: HClO ₂ + N(CH ₃) ₃ HN(CH ₃) ₃+ + ClO ₂ 1 -Which two substances represent Bronsted-Lowery bases in this reaction?</strong> A) HClO<sub>2</sub> and N(CH<sub>3</sub>)<sub>3</sub> B) HClO<sub>2</sub> and [HN(CH<sub>3</sub>)<sub>3</sub>]<sup>+</sup> C) N(CH<sub>3</sub>)<sub>3</sub> and [HN(CH<sub>3</sub>)<sub>3</sub>]<sup>+</sup> D) N(CH<sub>3</sub>)<sub>3</sub> and ClO<sub>2</sub><sup>1 - </sup> E) [HN(CH<sub>3</sub>)<sub>3</sub>]<sup>+ </sup>and ClO<sub>2</sub><sup>1 - </sup>](https://storage.examlex.com/TBX8714/11ebff50_c8bc_6b90_8da6_cb6cd585499a_TBX8714_11.jpg) HN(CH ₃) ₃+ + ClO ₂ 1 -Which two substances represent Bronsted-Lowery bases in this reaction?
HN(CH ₃) ₃+ + ClO ₂ 1 -Which two substances represent Bronsted-Lowery bases in this reaction?
A) HClO2 and N(CH3)3
B) HClO2 and [HN(CH3)3]+
C) N(CH3)3 and [HN(CH3)3]+
D) N(CH3)3 and ClO21 -
E) [HN(CH3)3]+ and ClO21 -
HClO ₂ + N(CH ₃) ₃
![<strong>Consider the following Bronsted-Lowery acid-base reaction: HClO ₂ + N(CH ₃) ₃ HN(CH ₃) ₃+ + ClO ₂ 1 -Which two substances represent Bronsted-Lowery bases in this reaction?</strong> A) HClO<sub>2</sub> and N(CH<sub>3</sub>)<sub>3</sub> B) HClO<sub>2</sub> and [HN(CH<sub>3</sub>)<sub>3</sub>]<sup>+</sup> C) N(CH<sub>3</sub>)<sub>3</sub> and [HN(CH<sub>3</sub>)<sub>3</sub>]<sup>+</sup> D) N(CH<sub>3</sub>)<sub>3</sub> and ClO<sub>2</sub><sup>1 - </sup> E) [HN(CH<sub>3</sub>)<sub>3</sub>]<sup>+ </sup>and ClO<sub>2</sub><sup>1 - </sup>](https://storage.examlex.com/TBX8714/11ebff50_c8bc_6b90_8da6_cb6cd585499a_TBX8714_11.jpg) HN(CH ₃) ₃+ + ClO ₂ 1 -Which two substances represent Bronsted-Lowery bases in this reaction?
HN(CH ₃) ₃+ + ClO ₂ 1 -Which two substances represent Bronsted-Lowery bases in this reaction?A) HClO2 and N(CH3)3
B) HClO2 and [HN(CH3)3]+
C) N(CH3)3 and [HN(CH3)3]+
D) N(CH3)3 and ClO21 -
E) [HN(CH3)3]+ and ClO21 -

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
6
What is the conjugate acid of HSO41 - ?
A) H2SO4
B) H2SO42 -
C) SO4
D) SO42 -
E) H3O+
A) H2SO4
B) H2SO42 -
C) SO4
D) SO42 -
E) H3O+

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
7
In the unusual acid-base reaction HClO₄ + H₂ SO₄ 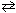 ClO₄- + H ₃SO₄+ , one of the conjugate acid-base pairs is:
ClO₄- + H ₃SO₄+ , one of the conjugate acid-base pairs is:
A) HClO4, H2SO4
B) H2SO4, ClO4 -
C) ClO4 - , H3SO4+
D) H3SO4+, H2SO4
E) HClO4, H3SO4+
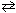 ClO₄- + H ₃SO₄+ , one of the conjugate acid-base pairs is:
ClO₄- + H ₃SO₄+ , one of the conjugate acid-base pairs is:A) HClO4, H2SO4
B) H2SO4, ClO4 -
C) ClO4 - , H3SO4+
D) H3SO4+, H2SO4
E) HClO4, H3SO4+

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
8
Which aqueous solution(s) below is(are) considered basic ?
I. A solution with a pH = 6
II. A solution with [OH - ] = 1×10 - 7
III. A solution with [H3O+] = 1×10 - 9
A) I only
B) II only
C) III only
D) I and II
E) I and III
I. A solution with a pH = 6
II. A solution with [OH - ] = 1×10 - 7
III. A solution with [H3O+] = 1×10 - 9
A) I only
B) II only
C) III only
D) I and II
E) I and III

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following aqueous solutions is(are) considered basic ?
I. [H3O+] = 5.4×10 - 8 M
II. pOH = 9.0
III. [OH - ] = 4.3×10 - 4
A) I only
B) I and II
C) I and III
D) II and III
E) All of these
I. [H3O+] = 5.4×10 - 8 M
II. pOH = 9.0
III. [OH - ] = 4.3×10 - 4
A) I only
B) I and II
C) I and III
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
10
What is the conjugate acid of HBO32 - ?
A) H3BO3
B) H2BO31 -
C) H2BO33 -
D) BO33 -
E) BO31 -
A) H3BO3
B) H2BO31 -
C) H2BO33 -
D) BO33 -
E) BO31 -

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
11
Which species listed below is the conjugate base of NH3?
A) NH4+
B) NH4
C) NH21+
D) NH21 -
E) OH -
A) NH4+
B) NH4
C) NH21+
D) NH21 -
E) OH -

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
12
The conjugate acid of HPO42 - is:
A) H3PO4
B) H2PO4 -
C) PO43 -
D) H+
E) none of these
A) H3PO4
B) H2PO4 -
C) PO43 -
D) H+
E) none of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
13
In the reaction:
N₂H4 (aq) + H₂ O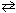 N₂H5 + (aq) + OH - (aq)
N₂H5 + (aq) + OH - (aq)
A) N2H4 and N2H5+ act as acids.
B) H2O and N2H5+ act as acids.
C) N2H5+ and OH - act as acids.
D) only OH - acts as a base.
E) none of these.
N₂H4 (aq) + H₂ O
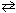 N₂H5 + (aq) + OH - (aq)
N₂H5 + (aq) + OH - (aq)A) N2H4 and N2H5+ act as acids.
B) H2O and N2H5+ act as acids.
C) N2H5+ and OH - act as acids.
D) only OH - acts as a base.
E) none of these.

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
14
Consider the following Bronsted-Lowry acid-base reaction:
C6H5NH2 + HCOOH→C6H5NH3+ + HCOO - Which of the following is a conjugate acid-base pair?
A) C6H5NH2 and HCO2H
B) HCOOH and C6H5NH3+
C) HCOO - and C6H5NH2
D) C6H5NH3+ and C6H5NH2
E) C6H5NH3+ and HCOO -
C6H5NH2 + HCOOH→C6H5NH3+ + HCOO - Which of the following is a conjugate acid-base pair?
A) C6H5NH2 and HCO2H
B) HCOOH and C6H5NH3+
C) HCOO - and C6H5NH2
D) C6H5NH3+ and C6H5NH2
E) C6H5NH3+ and HCOO -

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
15
At 37 ° C the autoionization constant of water is K w = 2.4×10 - 14. What is the pH of pure water at 37 ° C?
A) 6.81
B) 7.00
C) 7.19
D) 2.39
E) 11.61
A) 6.81
B) 7.00
C) 7.19
D) 2.39
E) 11.61

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
16
A Bronsted-Lowry acid is
A) a proton acceptor.
B) an electron acceptor.
C) an electron donor.
D) a binary hydride.
E) a proton donor
A) a proton acceptor.
B) an electron acceptor.
C) an electron donor.
D) a binary hydride.
E) a proton donor

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
17
What is the conjugate acid of the species, HAsO42 - ?
A) H3O+
B) H3AsO4
C) H2AsO41 -
D) H2AsO43 -
E) AsO43 -
A) H3O+
B) H3AsO4
C) H2AsO41 -
D) H2AsO43 -
E) AsO43 -

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
18
In the acid-base equilibrium PO4 3 - + NH ₄+ 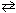 HPO3 2 - + NH₃, one conjugate acid-base pair is:
HPO3 2 - + NH₃, one conjugate acid-base pair is:
A) PO43 - , NH4+
B) NH4+ , HPO42 -
C) HPO42 - , NH3
D) NH4+ , NH3
E) none of these
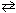 HPO3 2 - + NH₃, one conjugate acid-base pair is:
HPO3 2 - + NH₃, one conjugate acid-base pair is:A) PO43 - , NH4+
B) NH4+ , HPO42 -
C) HPO42 - , NH3
D) NH4+ , NH3
E) none of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
19
If the reaction below proceeds, which of the following is true?
NH3 + C2H5OH→NH2 - + C2H5OH2+
A) C2H5OH is an acid.
B) NH2 - is the conjugate base of NH3.
C) NH3 is a base.
D) C2H5OH2+ is a base.
E) none of these
NH3 + C2H5OH→NH2 - + C2H5OH2+
A) C2H5OH is an acid.
B) NH2 - is the conjugate base of NH3.
C) NH3 is a base.
D) C2H5OH2+ is a base.
E) none of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following aqueous solutions is(are) considered acidic ?
I. pOH = 12.0
II. [H3O+] = 4.2×10 - 9
III. [OH - ] = 5.3×10 - 10
A) II only
B) I and II
C) I and III
D) II and III
E) All of these
I. pOH = 12.0
II. [H3O+] = 4.2×10 - 9
III. [OH - ] = 5.3×10 - 10
A) II only
B) I and II
C) I and III
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
21
From the information given below which one of the 5 solutions would be the most acidic?
A) pH = 6
B) pOH = 9
C) [H3O+] = 10 - 3 M
D) [OH - ] = 10 - 10
E) pOH = 12
A) pH = 6
B) pOH = 9
C) [H3O+] = 10 - 3 M
D) [OH - ] = 10 - 10
E) pOH = 12

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
22
The pH of a certain black coffee is 5.3. What is its hydroxide ion concentration, [OH - ]?
A) 2.0×10 - 9 M
B) 5.0×10 - 6 M
C) 8.7 M
D) 2.0×105 M
E) 5.0×108 M
A) 2.0×10 - 9 M
B) 5.0×10 - 6 M
C) 8.7 M
D) 2.0×105 M
E) 5.0×108 M

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
23
Exhibit 15-2 The pH of a solution of a certain brand of Cola is 2.27. Use this information to solve the following problem(s).
Refer to Exhibit 15-2. What is the hydroxide ion concentration, [OH - ], of this solution?
A) 9.68×10 - 14 M
B) 1.86×10 - 12 M
C) 5.37×10 - 3 M
D) 1.03×10 - 1 M
E) 5.37×1011 M
Refer to Exhibit 15-2. What is the hydroxide ion concentration, [OH - ], of this solution?
A) 9.68×10 - 14 M
B) 1.86×10 - 12 M
C) 5.37×10 - 3 M
D) 1.03×10 - 1 M
E) 5.37×1011 M

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
24
What is the H3O+ ion concentration in an aqueous solution having an OH - concentration of 1.0×10 - 2 M?
A) 1.0×10 - 2 M
B) 13×10 - 2 M
C) 12×10 - 2 M
D) 1.0×10 - 12 M
E) 12×10 - 12 M
A) 1.0×10 - 2 M
B) 13×10 - 2 M
C) 12×10 - 2 M
D) 1.0×10 - 12 M
E) 12×10 - 12 M

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following aqueous solutions is(are) considered basic ?
I. [H3O+] = 3.4×10 - 10 M
II. pH = 8.54
III. [OH - ] = 6.7×10 - 4 M
A) III only
B) I and II
C) I and III
D) II and III
E) All of these
I. [H3O+] = 3.4×10 - 10 M
II. pH = 8.54
III. [OH - ] = 6.7×10 - 4 M
A) III only
B) I and II
C) I and III
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following aqueous solutions is(are) considered acidic ?
I. A solution with [OH - ] = 2.5×10 - 8
II. A solution with [H3O+] = 1×10 - 7
III. A solution with a pOH = 8
A) I only
B) II only
C) III only
D) I and II
E) I and III
I. A solution with [OH - ] = 2.5×10 - 8
II. A solution with [H3O+] = 1×10 - 7
III. A solution with a pOH = 8
A) I only
B) II only
C) III only
D) I and II
E) I and III

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
27
The pH of a diet Coca Cola solution is 3.12. What is the concentration of [OH - ] present in this solution?
A) [OH - ] = 1.3×10 - 25 M
B) [OH - ] = 7.6×10 - 18 M
C) [OH - ] = 1.3×10 - 11 M
D) [OH - ] = 7.6×10 - 4 M
E) [OH - ] = 7.6×1010 M
A) [OH - ] = 1.3×10 - 25 M
B) [OH - ] = 7.6×10 - 18 M
C) [OH - ] = 1.3×10 - 11 M
D) [OH - ] = 7.6×10 - 4 M
E) [OH - ] = 7.6×1010 M

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
28
A solution is found to have a pH of 10.45. What is [H3O+]?
A) 2.9×10 - 5 M
B) 1.0 M
C) 2.8×10 - 10 M
D) 3.5×10 - 11 M
E) cannot be calculated from the information given
A) 2.9×10 - 5 M
B) 1.0 M
C) 2.8×10 - 10 M
D) 3.5×10 - 11 M
E) cannot be calculated from the information given

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
29
The pH of a vinegar solution is 2.32. What is the hydronium ion concentration, [H3O+], for this solution?
A) [H3O+] = 2.1×10 - 12 M
B) [H3O+] = 4.8×10 - 3 M
C) [H3O+] = 0.36 M
D) [H3O+] = 2.3 M
E) [H3O+] = 2.1×102 M
A) [H3O+] = 2.1×10 - 12 M
B) [H3O+] = 4.8×10 - 3 M
C) [H3O+] = 0.36 M
D) [H3O+] = 2.3 M
E) [H3O+] = 2.1×102 M

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
30
Exhibit 15-1 The pH of a certain brand of milk is 6.60. Use this information to answer the following question(s).
Refer to Exhibit 15-1. What is the molar concentration of the hydronium ion , [H3O+], in this milk?
A) 2.5×10 - 7 M
B) 6.0×10 - 6 M
C) 6.6×10 - 1 M
D) 8.2×10 - 1 M
E) 4.0×106 M
Refer to Exhibit 15-1. What is the molar concentration of the hydronium ion , [H3O+], in this milk?
A) 2.5×10 - 7 M
B) 6.0×10 - 6 M
C) 6.6×10 - 1 M
D) 8.2×10 - 1 M
E) 4.0×106 M

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
31
Measurements show that the [OH - ] of a solution is 6.02×10 - 6 M. The pH of this solution is:
A) 8.78
B) 5.22
C) 6.02
D) 9.78
E) none of these
A) 8.78
B) 5.22
C) 6.02
D) 9.78
E) none of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
32
The hydroxide ion concentration in a solution with pH = 3.50 is:
A) 3.2×10 - 2 M
B) 3.2×10 - 4 M
C) 3.2×10 - 11 M
D) 3.2×10 - 10 M
E) none of these
A) 3.2×10 - 2 M
B) 3.2×10 - 4 M
C) 3.2×10 - 11 M
D) 3.2×10 - 10 M
E) none of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
33
The hydronium ion concentration, [H3O+], for a certain brand of beer is 3.16×10 - 5 M. What is the pOH of this aqueous solution?
A) pOH = 0.00; there can be no hydroxide ion present when hydronium ion is present.
B) pOH = 3.64
C) pOH = 4.50
D) pOH = 9.50
E) pOH = 10.36
A) pOH = 0.00; there can be no hydroxide ion present when hydronium ion is present.
B) pOH = 3.64
C) pOH = 4.50
D) pOH = 9.50
E) pOH = 10.36

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
34
Exhibit 15-2 The pH of a solution of a certain brand of Cola is 2.27. Use this information to solve the following problem(s).
Refer to Exhibit 15-2. What is the pOH of this solution?
A) 0.99
B) 6.17
C) 11.73
D) 16.27
E) 27.01
Refer to Exhibit 15-2. What is the pOH of this solution?
A) 0.99
B) 6.17
C) 11.73
D) 16.27
E) 27.01

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
35
The pH of a solution is 5. The [H3O+] and [OH - ] are:
A) 10 - 5 and 10 - 7 M, respectively.
B) 10 - 5 and 10 - 9 M, respectively.
C) 10 - 9 and 10 - 7 M, respectively.
D) 10 - 9 and 10 - 5 M, respectively.
E) none of these
A) 10 - 5 and 10 - 7 M, respectively.
B) 10 - 5 and 10 - 9 M, respectively.
C) 10 - 9 and 10 - 7 M, respectively.
D) 10 - 9 and 10 - 5 M, respectively.
E) none of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
36
The pH of a certain specimen of urine is 4.8. What is the hydroxide ion concentration, [OH - ], of this solution?
A) [OH - ] = 1.6×10 - 19 M
B) [OH - ] = 6.3×10 - 10 M
C) [OH - ] = 1.6×10 - 5 M
D) [OH - ] = 6.3×104 M
E) [OH - ] = 1.6×109 M
A) [OH - ] = 1.6×10 - 19 M
B) [OH - ] = 6.3×10 - 10 M
C) [OH - ] = 1.6×10 - 5 M
D) [OH - ] = 6.3×104 M
E) [OH - ] = 1.6×109 M

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
37
If the pH of a solution is 4.40:
A) [H3O+] = 4.0×10 - 5 M
B) [H3O+] = 2.5×10 - 4 M
C) [OH - ] = 3.4×10 - 9 M
D) [OH - ] = 2.5×10 - 11 M
E) pOH = 18.80
A) [H3O+] = 4.0×10 - 5 M
B) [H3O+] = 2.5×10 - 4 M
C) [OH - ] = 3.4×10 - 9 M
D) [OH - ] = 2.5×10 - 11 M
E) pOH = 18.80

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following aqueous solutions is(are) considered basic ?
I. [OH - ] = 6.2×10 - 8
II. [H3O+] = 3.8×10 - 12
III. pOH = 2.5
A) I only
B) I and II
C) I and III
D) II and III
E) All of these
I. [OH - ] = 6.2×10 - 8
II. [H3O+] = 3.8×10 - 12
III. pOH = 2.5
A) I only
B) I and II
C) I and III
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
39
Exhibit 15-1 The pH of a certain brand of milk is 6.60. Use this information to answer the following question(s).
Refer to Exhibit 15-1. What is the molar concentration of hydroxide ion , [OH - ], in this milk?
A) 2.5×10 - 21 M
B) 1.2×10 - 14 M
C) 1.5×10 - 14 M
D) 1.7×10 - 9 M
E) 4.0×10 - 8 M
Refer to Exhibit 15-1. What is the molar concentration of hydroxide ion , [OH - ], in this milk?
A) 2.5×10 - 21 M
B) 1.2×10 - 14 M
C) 1.5×10 - 14 M
D) 1.7×10 - 9 M
E) 4.0×10 - 8 M

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
40
A famous brand of sweet and sour barbecue sauce (consisting of tomato paste, water, vinegar, sugar, and secret spices) has a measured pH of 2.6. What is the hydrogen ion concentration in the sauce?
A) 0.42 M
B) 2.4×10 - 2 M
C) 2.5×10 - 3 M
D) 3.4×10 - 4 M
E) 5×10 - 5 M
A) 0.42 M
B) 2.4×10 - 2 M
C) 2.5×10 - 3 M
D) 3.4×10 - 4 M
E) 5×10 - 5 M

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
41
A solution of KOH is found to have [OH - ] = 0.010 M. What is the pH of this solution?
A) 14.0
B) 12.0
C) 2.0
D) 13.0
E) 1.0
A) 14.0
B) 12.0
C) 2.0
D) 13.0
E) 1.0

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
42
Which compound is a strong acid in water?
A) H2SO4
B) NH3
C) HF
D) NaOH
E) none of these
A) H2SO4
B) NH3
C) HF
D) NaOH
E) none of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
43
What is the Calcium hydroxide concentration, [Ca(OH)2], of an aqueous Ca(OH)2 solution with a pH = 9.60?
A) 1.3×10 - 10 M
B) 2.5×10 - 10 M
C) 2.0×10 - 5 M
D) 4.0×10 - 5 M
E) 8.0×10 - 5 M
A) 1.3×10 - 10 M
B) 2.5×10 - 10 M
C) 2.0×10 - 5 M
D) 4.0×10 - 5 M
E) 8.0×10 - 5 M

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
44
Acid strengths are measured and compared how?
A) By measuring the concentration of the acid.
B) By measuring the pH of the aqueous solution.
C) By measuring the extent of ionization as determined by the reaction's K a.
D) By measuring how corrosive the acid is.
E) All of these.
A) By measuring the concentration of the acid.
B) By measuring the pH of the aqueous solution.
C) By measuring the extent of ionization as determined by the reaction's K a.
D) By measuring how corrosive the acid is.
E) All of these.

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
45
What is the pH of 2.0 liters of aqueous solution that contains 9.0 grams of HCl?
A) 0.12
B) 0.660
C) 0.810
D) 0.90
E) 13.10
A) 0.12
B) 0.660
C) 0.810
D) 0.90
E) 13.10

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
46
What is the pH of 0.0040 M HCl ?
A) 3.61
B) 11.60
C) 10.63
D) 2.64
E) 2.40
A) 3.61
B) 11.60
C) 10.63
D) 2.64
E) 2.40

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is considered a strong acid when dissolved in water?
A) H3PO4
B) HF
C) H2SO4
D) HClO
E) All of these
A) H3PO4
B) HF
C) H2SO4
D) HClO
E) All of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
48
A nitric acid (HNO3) solution is 16.2 M. Which of the following is correct?
A) pH = 1.21
B) pH = 2.78
C) pOH = 15.21
D) [OH - ] = 1.62×1015
E) must know K a
A) pH = 1.21
B) pH = 2.78
C) pOH = 15.21
D) [OH - ] = 1.62×1015
E) must know K a

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following acids is considered a strong acid when dissolved in water?
I. HNO2 (Nitrous acid)
II. HClO4 (Perchloric acid)
III. HF (Hydrofluoric acid)
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. HNO2 (Nitrous acid)
II. HClO4 (Perchloric acid)
III. HF (Hydrofluoric acid)
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
50
What is the pOH of a 1.0×10 - 4 M solution of HI?
A) 10.00
B) 4.00
C) 18.00
D) - 4.00
E) 2.00
A) 10.00
B) 4.00
C) 18.00
D) - 4.00
E) 2.00

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is considered a weak acid when dissolved in water?
I. HNO2
II. HF
III. H2SO3
A) I only
B) II only
C) III only
D) All of these
E) None of these
I. HNO2
II. HF
III. H2SO3
A) I only
B) II only
C) III only
D) All of these
E) None of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
52
What is the pH of an aqueous 3.3 M Ba(OH)2 solution?
A) pH = - 0.51
B) pH = - 0.81
C) pH = 13.19
D) pH = 13.49
E) pH = 14.82
A) pH = - 0.51
B) pH = - 0.81
C) pH = 13.19
D) pH = 13.49
E) pH = 14.82

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
53
Given the following Bronsted-Lowery acid-base reaction and its corresponding equilibrium constant, which substance is the strongest acid ?
CH ₃CH ₂ NH₃+ + ClO21 -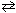 CH ₃CH ₂ NH₂ + HClO ₂ K = 2.1 10 - 9
CH ₃CH ₂ NH₂ + HClO ₂ K = 2.1 10 - 9
A) CH3CH2NH3+
B) ClO21 -
C) CH3CH2NH2
D) HClO2
CH ₃CH ₂ NH₃+ + ClO21 -
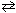 CH ₃CH ₂ NH₂ + HClO ₂ K = 2.1 10 - 9
CH ₃CH ₂ NH₂ + HClO ₂ K = 2.1 10 - 9A) CH3CH2NH3+
B) ClO21 -
C) CH3CH2NH2
D) HClO2

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
54
A dilute solution of HBr is found to have a pH of 3.55. What is [H3O+]?
A) 0.35 M
B) 3.8×10 - 4 M
C) 2.8×10 - 4 M
D) 5.5×10 - 3 M
E) 3.2×10 - 11 M
A) 0.35 M
B) 3.8×10 - 4 M
C) 2.8×10 - 4 M
D) 5.5×10 - 3 M
E) 3.2×10 - 11 M

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
55
What is the pH of 0.025 M Ba(OH)2?
A) 0.050
B) 1.30
C) 12.70
D) 2×10 - 13
E) none of these
A) 0.050
B) 1.30
C) 12.70
D) 2×10 - 13
E) none of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
56
What is the pH of a 2.6 M aqueous solution of Ba(OH)2?
A) 7.94×10 - 6
B) 0.71
C) 13.29
D) 14.41
E) 14.72
A) 7.94×10 - 6
B) 0.71
C) 13.29
D) 14.41
E) 14.72

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
57
What is the pH of 3.9×10 - 4 M HNO3?
A) - 3.40
B) 0.03
C) 3.41
D) 3.51
E) none of these
A) - 3.40
B) 0.03
C) 3.41
D) 3.51
E) none of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
58
Which compound is a strong base in water?
A) H2SO4
B) NH3
C) HF
D) NaOH
E) none of these
A) H2SO4
B) NH3
C) HF
D) NaOH
E) none of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is considered a strong acid when dissolved in water?
I. HClO2
II. HBr
III. HNO3
A) I only
B) II only
C) II and III
D) All of these
E) None of these
I. HClO2
II. HBr
III. HNO3
A) I only
B) II only
C) II and III
D) All of these
E) None of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
60
What is the pH of 1×10 - 6 M HCl?
A) 6.00
B) 6.98
C) 7.98
D) 8.00
E) need K a
A) 6.00
B) 6.98
C) 7.98
D) 8.00
E) need K a

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
61
What is the H3O+ concentration in a solution formed by dissolving 63 g of HNO3 in 2.0 liters of water?
[molar mass of HNO3 = 63 g/mol]
A) 1.0 M
B) 0.50 M
C) 0.050 M
D) 2.0 M
E) none of these
[molar mass of HNO3 = 63 g/mol]
A) 1.0 M
B) 0.50 M
C) 0.050 M
D) 2.0 M
E) none of these

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
62
Which base listed below would be the strongest given the corresponding acid ionization constants for their conjugate acids?
A) PO43 - ; K a(HPO41 - ) = 4.2×10 - 13
B) NO21 - ; K a(HNO2) = 7.1×10 - 4
C) HCO31 - ; K a(H2CO3) = 4.5×10 - 7
D) CH3COO1 - ; K a(CH3COOH) = 1.8×10 - 5
E) F - ; K a(HF) = 6.8×10 - 4
A) PO43 - ; K a(HPO41 - ) = 4.2×10 - 13
B) NO21 - ; K a(HNO2) = 7.1×10 - 4
C) HCO31 - ; K a(H2CO3) = 4.5×10 - 7
D) CH3COO1 - ; K a(CH3COOH) = 1.8×10 - 5
E) F - ; K a(HF) = 6.8×10 - 4

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
63
Consider the following unfinished table of weak acids with their corresponding
P K a values and K a values. Weak Acid p K a K a HNO2 3.35 -- HF -- 6.8×10 - 4 HCN 9.31 --
Arrange these three acids in order of increasing acid strength .
A) (weakest) HNO2 < HF < HCN (strongest)
B) (weakest) HNO2 < HCN < HF (strongest)
C) (weakest) HCN < HF < HNO2 (strongest)
D) (weakest) HCN < HNO2 < HF (strongest)
E) (weakest) HF < HNO2 < HCN (strongest)
P K a values and K a values. Weak Acid p K a K a HNO2 3.35 -- HF -- 6.8×10 - 4 HCN 9.31 --
Arrange these three acids in order of increasing acid strength .
A) (weakest) HNO2 < HF < HCN (strongest)
B) (weakest) HNO2 < HCN < HF (strongest)
C) (weakest) HCN < HF < HNO2 (strongest)
D) (weakest) HCN < HNO2 < HF (strongest)
E) (weakest) HF < HNO2 < HCN (strongest)

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
64
After completing the following unfinished Base Ionization table, what is the order of increasing base strength ?
Base K b p K b Bicarbonate ion, HCO3 - 2.2×10 - 8 Pyridine, C5H5N 8.77 Acetate ion, CH3COO - 5.7×10 - 10
A) (weakest) Bicarbonate ion < Pyridine < Acetate ion (strongest)
B) (weakest) Bicarbonate ion < Acetate ion < Pyridine (strongest)
C) (weakest) Pyridine < Bicarbonate ion < Acetate ion (strongest)
D) (weakest) Acetate ion < Pyridine < Bicarbonate ion (strongest)
E) (weakest) Acetate ion < Bicarbonate ion < Pyridine (strongest)
Base K b p K b Bicarbonate ion, HCO3 - 2.2×10 - 8 Pyridine, C5H5N 8.77 Acetate ion, CH3COO - 5.7×10 - 10
A) (weakest) Bicarbonate ion < Pyridine < Acetate ion (strongest)
B) (weakest) Bicarbonate ion < Acetate ion < Pyridine (strongest)
C) (weakest) Pyridine < Bicarbonate ion < Acetate ion (strongest)
D) (weakest) Acetate ion < Pyridine < Bicarbonate ion (strongest)
E) (weakest) Acetate ion < Bicarbonate ion < Pyridine (strongest)

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
65
Milk of magnesia is essentially a saturated solution of Mg(OH)2 ( K sp = 8.9×10 - 12). What is the pH of a saturated Mg(OH)2 solution?
A) 10.32
B) 5.52
C) 10.12
D) 10.42
E) >12.0
A) 10.32
B) 5.52
C) 10.12
D) 10.42
E) >12.0

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
66
What is the pH of 2.7 M Ba(OH)2?
A) pH = - 0.726
B) pH = 0.425
C) pH = 5.32
D) pH = 13.27
E) pH = 14.73
A) pH = - 0.726
B) pH = 0.425
C) pH = 5.32
D) pH = 13.27
E) pH = 14.73

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
67
50 mL of 0.50 M NaOH is added to enough water to make 1.0 liter of solution. Calculate the pH.
A) 0.3
B) 1.6
C) 12.4
D) 12.7
E) 13.7
A) 0.3
B) 1.6
C) 12.4
D) 12.7
E) 13.7

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
68
What is the pH of a solution prepared by dissolving 0.28 grams of KOH in 250 mL of solution?
(molar mass KOH = 56.11 g/mol)
A) 1.70
B) 12.30
C) 2.00×10 - 2
D) 11.7
E) 5.0×10 - 13
(molar mass KOH = 56.11 g/mol)
A) 1.70
B) 12.30
C) 2.00×10 - 2
D) 11.7
E) 5.0×10 - 13

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
69
A weak acid
A) does not react with base
B) does not react with glass
C) does not burn skin
D) does not dissociate fully
E) does not pose a disposal problem
A) does not react with base
B) does not react with glass
C) does not burn skin
D) does not dissociate fully
E) does not pose a disposal problem

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
70
Which anionic base listed below would be the strongest given the corresponding acid ionization constants for their conjugate acids?
A) F - ; K a(HF) = 6.8×10 - 4
B) CH3COO - ; K a(CH3COOH) = 1.8×10 - 5
C) IO31 - ; K a(HIO3) = 1.6×10 - 1
D) NO21 - ; K a(HNO2) = 7.1×10 - 4
A) F - ; K a(HF) = 6.8×10 - 4
B) CH3COO - ; K a(CH3COOH) = 1.8×10 - 5
C) IO31 - ; K a(HIO3) = 1.6×10 - 1
D) NO21 - ; K a(HNO2) = 7.1×10 - 4

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
71
A 0.010 M solution of the strong acid HNO3 is prepared. Which of the following statements is false ?
A) The acid is completely ionized.
B) The pH of the solution is 2.0.
C) The equilibrium [NO3 - ] is approximately 0.010 M.
D) The aqueous NO3 - ion is a very weak base.
E) The equilibrium [HNO3] is approximately 0.010 M.
A) The acid is completely ionized.
B) The pH of the solution is 2.0.
C) The equilibrium [NO3 - ] is approximately 0.010 M.
D) The aqueous NO3 - ion is a very weak base.
E) The equilibrium [HNO3] is approximately 0.010 M.

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
72
What is the pH of a solution prepared by dissolving 0.13 grams of HI (molar mass HI = 127.9 g/mol) in 300 mL of solution?
A) 5.48
B) 2.47
C) 0.37
D) 3.37
E) not enough information is given
A) 5.48
B) 2.47
C) 0.37
D) 3.37
E) not enough information is given

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
73
Rank the following three acids in terms of acid strength given the following information:
Acid K a p K a HF 6.9×10 - 4 -- CH3COOH -- 4.74 HNO2 4.5×10 - 4
A) (weakest acid) HF < CH3COOH < HNO2 (strongest acid)
B) (weakest acid) CH3COOH < HNO2 < HF (strongest acid)
C) (weakest acid) HNO2 < HF < CH3COOH (strongest acid)
D) (weakest acid) HF < HNO2 < CH3COOH (strongest acid)
E) (weakest acid) HNO2 < CH3COOH < HF (strongest acid)
Acid K a p K a HF 6.9×10 - 4 -- CH3COOH -- 4.74 HNO2 4.5×10 - 4
A) (weakest acid) HF < CH3COOH < HNO2 (strongest acid)
B) (weakest acid) CH3COOH < HNO2 < HF (strongest acid)
C) (weakest acid) HNO2 < HF < CH3COOH (strongest acid)
D) (weakest acid) HF < HNO2 < CH3COOH (strongest acid)
E) (weakest acid) HNO2 < CH3COOH < HF (strongest acid)

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
74
According to the definition of pH, what would be the pH of a 10 M solution of HCl?
A) 1
B) 0
C) - 1
D) 0.30
E) - 0.30
A) 1
B) 0
C) - 1
D) 0.30
E) - 0.30

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
75
What is the pH of a 1.6 M aqueous solution of Sr(OH)2?
A) pH = - 0.49
B) pH = 12.87
C) pH = 13.81
D) pH = 14.19
E) pH = 14.50
A) pH = - 0.49
B) pH = 12.87
C) pH = 13.81
D) pH = 14.19
E) pH = 14.50

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
76
Consider the following unfinished table of weak acids with their corresponding p K a values and K a values. Weak Acid p K a K a Benzoic acid 4.20 -- Lactic acid -- 1.4×10 - 4 Chlorous acid 1.96 -- Arrange these three acids in order of increasing acid strength .
A) (weakest) Benzoic acid < Lactic acid < Chlorous acid (strongest)
B) (weakest) Benzoic acid < Chlorous acid < Lactic acid (strongest)
C) (weakest) Lactic acid < Benzoic acid < Chlorous acid (strongest)
D) (weakest) Lactic acid < Chlorous acid < Benzoic acid (strongest)
E) (weakest) Chlorous acid < Benzoic acid < Lactic acid (strongest)
A) (weakest) Benzoic acid < Lactic acid < Chlorous acid (strongest)
B) (weakest) Benzoic acid < Chlorous acid < Lactic acid (strongest)
C) (weakest) Lactic acid < Benzoic acid < Chlorous acid (strongest)
D) (weakest) Lactic acid < Chlorous acid < Benzoic acid (strongest)
E) (weakest) Chlorous acid < Benzoic acid < Lactic acid (strongest)

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
77
Calculate the pOH of a solution made by dissolving 2.0 grams of NaOH (molar mass = 40.0 g/mol) in water and diluting tO5 00 mL.
A) 1.00
B) 13.00
C) 1.12
D) 1.30
E) 12.70
A) 1.00
B) 13.00
C) 1.12
D) 1.30
E) 12.70

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
78
Which acid listed below would be the strongest given the corresponding base ionization constants for their conjugate bases?
A) H3PO4; K b(H2PO41 - ) = 1.4×10 - 12
B) H2S; K b(HS - ) = 1.1×10 - 7
C) HClO2; K b(ClO2 - ) = 9.1×10 - 13
D) HNO2; K b(NO2 - ) = 1.4×10 - 11
E) HIO; K b(IO - ) = 4.3×10 - 4
A) H3PO4; K b(H2PO41 - ) = 1.4×10 - 12
B) H2S; K b(HS - ) = 1.1×10 - 7
C) HClO2; K b(ClO2 - ) = 9.1×10 - 13
D) HNO2; K b(NO2 - ) = 1.4×10 - 11
E) HIO; K b(IO - ) = 4.3×10 - 4

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
79
After completing the following unfinished table, what is the order of increasing base strength ?
Base K b p K b Fluoride ion, F - 1.5×10 - 11 Ammonia, NH3 4.75 Acetate ion, CH3COO - 5.7×10 - 10
A) (weakest) Fluoride ion < Ammonia < Acetate ion (strongest)
B) (weakest) Fluoride ion < Acetate ion < Ammonia (strongest)
C) (weakest) Ammonia < Fluoride ion < Acetate ion (strongest)
D) (weakest) Acetate ion < Ammonia < Fluoride ion (strongest)
E) (weakest) Acetate ion < Fluoride ion < Ammonia (strongest)
Base K b p K b Fluoride ion, F - 1.5×10 - 11 Ammonia, NH3 4.75 Acetate ion, CH3COO - 5.7×10 - 10
A) (weakest) Fluoride ion < Ammonia < Acetate ion (strongest)
B) (weakest) Fluoride ion < Acetate ion < Ammonia (strongest)
C) (weakest) Ammonia < Fluoride ion < Acetate ion (strongest)
D) (weakest) Acetate ion < Ammonia < Fluoride ion (strongest)
E) (weakest) Acetate ion < Fluoride ion < Ammonia (strongest)

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck
80
After completing the following unfinished table, what is the order of increasing acid strength ?
Acid K a p K a Propanoic acid 1.3×10 - 5 Chlorous acid 1.96 Benzoic acid 6.3×10 - 5
A) (weakest) Propanoic acid < Chlorous acid < Benzoic acid (strongest)
B) (weakest) Propanoic acid < Benzoic acid < Chlorous acid (strongest)
C) (weakest) Chlorous acid < Propanoic acid < Benzoic acid (strongest)
D) (weakest) Benzoic acid < Chlorous acid < Propanoic acid (strongest)
E) (weakest) Benzoic acid < Propanoic acid < Chlorous acid (strongest)
Acid K a p K a Propanoic acid 1.3×10 - 5 Chlorous acid 1.96 Benzoic acid 6.3×10 - 5
A) (weakest) Propanoic acid < Chlorous acid < Benzoic acid (strongest)
B) (weakest) Propanoic acid < Benzoic acid < Chlorous acid (strongest)
C) (weakest) Chlorous acid < Propanoic acid < Benzoic acid (strongest)
D) (weakest) Benzoic acid < Chlorous acid < Propanoic acid (strongest)
E) (weakest) Benzoic acid < Propanoic acid < Chlorous acid (strongest)

Unlock Deck
Unlock for access to all 179 flashcards in this deck.
Unlock Deck
k this deck


