Deck 19: Transition Metals, Coordination Chemistry and Metallurgy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/73
Play
Full screen (f)
Deck 19: Transition Metals, Coordination Chemistry and Metallurgy
1
In coordination compounds, the term used to
Refer to the number of ligands bound to the metal is:
A) oxidation state.
B) complexation.
C) coordination number.
D) hydration number.
E) none of these.
Refer to the number of ligands bound to the metal is:
A) oxidation state.
B) complexation.
C) coordination number.
D) hydration number.
E) none of these.
coordination number.
2
Which metal has the largest heat of vaporization?
A) K
B) Na
C) Cu
D) Cr
E) Al
A) K
B) Na
C) Cu
D) Cr
E) Al
Cr
3
The name of the coordination compound Na[FeCl4] is:
A) sodium tetrachloroiron.
B) sodium tetrachloroferrate(III).
C) sodium tetrachloroironate(I).
D) sodium iron chloride.
E) none of these.
A) sodium tetrachloroiron.
B) sodium tetrachloroferrate(III).
C) sodium tetrachloroironate(I).
D) sodium iron chloride.
E) none of these.
sodium tetrachloroferrate(III).
4
What is the oxidation state of cobalt in the compound [Co(NH3)4Cl2]Cl?
A) +1
B) +2
C) +3
D) +6
E) none of these
A) +1
B) +2
C) +3
D) +6
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
5
What is the oxidation state of the ruthenium ion in the compound, K2[RuCl5(H2O)]?
A) - 2
B) 0
C) +1
D) +3
E) +5
A) - 2
B) 0
C) +1
D) +3
E) +5

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
6
Which of these metals is not a transition element?
A) Ti
B) Cr
C) Ca
D) La
E) Fe
A) Ti
B) Cr
C) Ca
D) La
E) Fe

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
7
Which of these transition elements has the largest atomic radius?
A) Ti
B) Fe
C) Mn
D) Hf
E) Re
A) Ti
B) Fe
C) Mn
D) Hf
E) Re

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
8
What is the coordination number of the central atom in the coordination compound [Co(en)2Br2]+?
(en = H2NCH2CH2NH2)
A) 4
B) 6
C) 8
D) 12
E) 26
(en = H2NCH2CH2NH2)
A) 4
B) 6
C) 8
D) 12
E) 26

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
9
The maximum oxidation state of a transition metal is usually observed when it is combined with:
A) another transition metal.
B) oxygen or fluorine.
C) alkali metals.
D) bromine or iodine.
E) none of these
A) another transition metal.
B) oxygen or fluorine.
C) alkali metals.
D) bromine or iodine.
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
10
Which metal listed below has the highest melting point?
A) Na
B) K
C) Cu
D) Cr
E) Ti
A) Na
B) K
C) Cu
D) Cr
E) Ti

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
11
The highest oxidation state expected for Ti is:
A) +1
B) +2
C) +3
D) +4
E) +5
A) +1
B) +2
C) +3
D) +4
E) +5

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
12
Given the three statements below, select the correct choice.
I. The lanthanide contraction causes Nb and Ta to have the same atomic radius.
II. The lanthanide contraction causes the radius of Zr to be greater than the radius of Ti.
III. The lanthanide contraction causes the first ionization energy of W to be larger than that of Mo.
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) only I is true
E) none of these
I. The lanthanide contraction causes Nb and Ta to have the same atomic radius.
II. The lanthanide contraction causes the radius of Zr to be greater than the radius of Ti.
III. The lanthanide contraction causes the first ionization energy of W to be larger than that of Mo.
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) only I is true
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
13
What is the oxidation state of the nickel ion in the compound [Ni(CO)4]?
A) - 4
B) - 2
C) 0
D) +2
E) +4
A) - 4
B) - 2
C) 0
D) +2
E) +4

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
14
What is the coordination number of the central atom in the coordination compound [Pt(NH3)4Cl2]SO4?
A) 6
B) 2
C) 3
D) 4
E) 18
A) 6
B) 2
C) 3
D) 4
E) 18

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
15
Which of these metals is not a transition element?
A) V
B) W
C) Fe
D) Cu
E) Al
A) V
B) W
C) Fe
D) Cu
E) Al

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
16
The formula of potassium pentacyanocarbonylcobaltate(III) is:
A) K3[Co(CN)5CO]
B) K2Co(CN)5 × CO
C) K2[Co(CN)5CO]
D) K4[Co(CN)5CO3]
E) none of these
A) K3[Co(CN)5CO]
B) K2Co(CN)5 × CO
C) K2[Co(CN)5CO]
D) K4[Co(CN)5CO3]
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the statements are true of ligands?
A) Ligands are Lewis bases.
B) Ligands are always anions.
C) Ligands only bond to a metal through a single atom.
D) Ligands accept electron pairs from the metal when forming bonds.
E) None of these.
A) Ligands are Lewis bases.
B) Ligands are always anions.
C) Ligands only bond to a metal through a single atom.
D) Ligands accept electron pairs from the metal when forming bonds.
E) None of these.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
18
Which of these transition elements has the largest atomic radius?
A) Cr
B) Co
C) Ta
D) Re
E) Os
A) Cr
B) Co
C) Ta
D) Re
E) Os

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
19
The highest oxidation state expected for Nb is:
A) +1
B) +2
C) +3
D) +4
E) +5
A) +1
B) +2
C) +3
D) +4
E) +5

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
20
Which of these metals is not a transition element?
A) Hf
B) Co
C) Cu
D) Zn
E) Pt
A) Hf
B) Co
C) Cu
D) Zn
E) Pt

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following ligands might form chelates with a metal ion?
A) Cl -
B) NH3
C) SCN -
D) C2O42 -
E) none of these
A) Cl -
B) NH3
C) SCN -
D) C2O42 -
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
22
What is the formula for the compound Sodium aquapentacyanocobaltate(III)?
A) Na[Co(CN)5] (aq)
B) Na2[Co(CN)5H2O]
C) Na3[Co(CN)5H2O]
D) Na[Co(CN)H2O]5
E) None of these
A) Na[Co(CN)5] (aq)
B) Na2[Co(CN)5H2O]
C) Na3[Co(CN)5H2O]
D) Na[Co(CN)H2O]5
E) None of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
23
What is the IUPAC name for the coordination compound with a formula of [Ni(CO)4]?
A) Nickel tetra(carbon monoxide)
B) Nickel tetracarbonyl
C) Tetracarbonylnickel(0)
D) Tetra(carbon monoxide)nickel(IV)
E) Tetra(Nickelcarbonyl)
A) Nickel tetra(carbon monoxide)
B) Nickel tetracarbonyl
C) Tetracarbonylnickel(0)
D) Tetra(carbon monoxide)nickel(IV)
E) Tetra(Nickelcarbonyl)

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
24
In which of the complexes are optical isomers possible?
A) [Cr(C2O4)3]3 -
B) [Co(en)3]3+
C) [Pt(NH3)2Cl2Br2]
D) none of these three
E) all of these three
A) [Cr(C2O4)3]3 -
B) [Co(en)3]3+
C) [Pt(NH3)2Cl2Br2]
D) none of these three
E) all of these three

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
25
How are the two compounds that are shown below related?
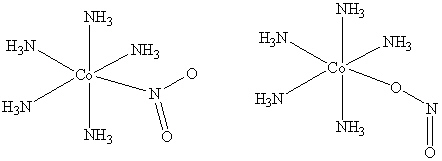
A) They are the same compound.
B) They are optical isomers.
C) They are structural coordination isomers.
D) They are structural linkage isomers.
E) They are geometric isomers.

A) They are the same compound.
B) They are optical isomers.
C) They are structural coordination isomers.
D) They are structural linkage isomers.
E) They are geometric isomers.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
26
Which pair of isomers is an example of coordination isomerism?
A) [Pt(NH3)4Br2]Cl2, [Pt(NH3)4Cl2]Br2
B)![<strong>Which pair of isomers is an example of coordination isomerism?</strong> A) [Pt(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]Cl<sub>2</sub>, [Pt(NH<sub>3</sub>)<sub>4</sub>Cl<sub>2</sub>]Br<sub>2</sub> B) C) cis -[Pt(NH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub>], trans -[Pt(NH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub>] D) E) none of these](https://storage.examlex.com/TBX8714/11ebff50_c8cf_cc08_8da6_d33b05a105d3_TBX8714_11.jpg)
C) cis -[Pt(NH3)2Cl2], trans -[Pt(NH3)2Cl2]
D)![<strong>Which pair of isomers is an example of coordination isomerism?</strong> A) [Pt(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]Cl<sub>2</sub>, [Pt(NH<sub>3</sub>)<sub>4</sub>Cl<sub>2</sub>]Br<sub>2</sub> B) C) cis -[Pt(NH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub>], trans -[Pt(NH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub>] D) E) none of these](https://storage.examlex.com/TBX8714/11ebff50_c8cf_cc09_8da6_c1a880836864_TBX8714_11.jpg)
E) none of these
A) [Pt(NH3)4Br2]Cl2, [Pt(NH3)4Cl2]Br2
B)
![<strong>Which pair of isomers is an example of coordination isomerism?</strong> A) [Pt(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]Cl<sub>2</sub>, [Pt(NH<sub>3</sub>)<sub>4</sub>Cl<sub>2</sub>]Br<sub>2</sub> B) C) cis -[Pt(NH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub>], trans -[Pt(NH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub>] D) E) none of these](https://storage.examlex.com/TBX8714/11ebff50_c8cf_cc08_8da6_d33b05a105d3_TBX8714_11.jpg)
C) cis -[Pt(NH3)2Cl2], trans -[Pt(NH3)2Cl2]
D)
![<strong>Which pair of isomers is an example of coordination isomerism?</strong> A) [Pt(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]Cl<sub>2</sub>, [Pt(NH<sub>3</sub>)<sub>4</sub>Cl<sub>2</sub>]Br<sub>2</sub> B) C) cis -[Pt(NH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub>], trans -[Pt(NH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub>] D) E) none of these](https://storage.examlex.com/TBX8714/11ebff50_c8cf_cc09_8da6_c1a880836864_TBX8714_11.jpg)
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following ligands is considered bidentate ?
I. H2O
II. oxalate, C2O42 -
III. ethylenediamine, H2NCH2CH2NH2
A) I only
B) II only
C) III only
D) I and II
E) II and III
I. H2O
II. oxalate, C2O42 -
III. ethylenediamine, H2NCH2CH2NH2
A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
28
What is the formula for the compound Triamminetrichlorocobalt(III)?
A) [Co(NH2)3]Cl3
B) Co(NH3)3Cl3
C) [Co(NH3)3Cl2]Cl
D) [Co(NH3)3Cl]Cl2
E) [Co(NH3)3]Cl3
A) [Co(NH2)3]Cl3
B) Co(NH3)3Cl3
C) [Co(NH3)3Cl2]Cl
D) [Co(NH3)3Cl]Cl2
E) [Co(NH3)3]Cl3

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following cannot behave as a ligand?
A) NH4+
B) H2O
C) CN -
D) OH -
E) C2O42 -
A) NH4+
B) H2O
C) CN -
D) OH -
E) C2O42 -

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
30
What is the IUPAC name for the coordination compound with a formula of [Co(NH3)5Br]Br2?
A) Pentaamminebromocobalt(III) bromide
B) Pentaamminetribromocobalt(III)
C) Pentaammoniacobalt(III) tribromide
D) Cobaltpentaamminebromo dibromide
E) Cobaltpentaammoniumbromo bromine
A) Pentaamminebromocobalt(III) bromide
B) Pentaamminetribromocobalt(III)
C) Pentaammoniacobalt(III) tribromide
D) Cobaltpentaamminebromo dibromide
E) Cobaltpentaammoniumbromo bromine

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
31
A ligand that forms complexes that exhibit linkage isomerism is:
A) Cl -
B) C2O4 -
C) ethylenediamine, [NH2(CH2)2NH2]
D) NO2 -
E) none of these
A) Cl -
B) C2O4 -
C) ethylenediamine, [NH2(CH2)2NH2]
D) NO2 -
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
32
How are the two compounds that are shown below related?
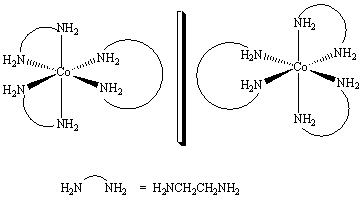
A) They are the same compound.
B) They are optical isomers.
C) They are structural coordination isomers.
D) They are structural linkage isomers.
E) They are geometric isomers.

A) They are the same compound.
B) They are optical isomers.
C) They are structural coordination isomers.
D) They are structural linkage isomers.
E) They are geometric isomers.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
33
How are the two compounds that are shown below related?
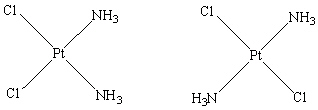
A) They are the same compound.
B) They are optical isomers.
C) They are structural coordination isomers.
D) They are structural linkage isomers.
E) They are geometric isomers.
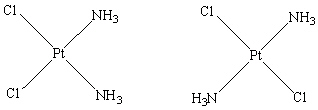
A) They are the same compound.
B) They are optical isomers.
C) They are structural coordination isomers.
D) They are structural linkage isomers.
E) They are geometric isomers.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
34
A coordination complex that can have two geometric isomers is:
A) [Co(NH3)5NO2]2+
B) [Co(en)2Cl2]+
C) [Pt(NH3)3SCN]+
D) [Cr(H2O)6]3+
E) none of these
A) [Co(NH3)5NO2]2+
B) [Co(en)2Cl2]+
C) [Pt(NH3)3SCN]+
D) [Cr(H2O)6]3+
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
35
How are the compounds [Co(NH3)4Cl2]Br and [Co(NH3)4BrCl]Cl related?
A) They are the same compound.
B) They are optical isomers.
C) They are structural coordination isomers.
D) They are structural linkage isomers.
E) They are geometric isomers.
A) They are the same compound.
B) They are optical isomers.
C) They are structural coordination isomers.
D) They are structural linkage isomers.
E) They are geometric isomers.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
36
How many geometric isomers are possible for the octahedral complex [Ru(H2O)2Cl4] - ?
A) 1
B) 2
C) 3
D) 4
E) 6
A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
37
What is the IUPAC name for the coordination compound with a formula of K3[Fe(CN)6]?
A) Tripotassium iron hexacyanide
B) Tripotassium iron(III) cyanide
C) Potassium hexacyanoferrate (III)
D) Potassium hexacyanoiron(III)
E) Tripotassium hexa(iron cyanide)
A) Tripotassium iron hexacyanide
B) Tripotassium iron(III) cyanide
C) Potassium hexacyanoferrate (III)
D) Potassium hexacyanoiron(III)
E) Tripotassium hexa(iron cyanide)

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
38
A complex that can exhibit both geometric and optical isomerism is:
A) [Co(en)3]3+
B) [Pt(NH3)2Cl2]
C) [Co(en)2Cl2]+
D) [Co(en)2(C2O4)]+
E) none of these
A) [Co(en)3]3+
B) [Pt(NH3)2Cl2]
C) [Co(en)2Cl2]+
D) [Co(en)2(C2O4)]+
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
39
The prefixes fac - and mer - are used to distinguish geometric isomers of octahedral complexes with which general formula?
(M = metal ion, A and B are monodentate ligands)
A) MA5B
B) MA4B2
C) MA3B3
D) all of these three
E) none of these three
(M = metal ion, A and B are monodentate ligands)
A) MA5B
B) MA4B2
C) MA3B3
D) all of these three
E) none of these three

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
40
Which coordination compound can exhibit geometric ( cis - trans ) isomerism?
A) [Pt(NH3)3Cl]+, square planar
B) [Zn(NH3)2Cl2], tetrahedral
C) [Pt(NH3)2Cl2], square planar
D) [Co(NH3)5Cl]+, octahedral
E) none of these
A) [Pt(NH3)3Cl]+, square planar
B) [Zn(NH3)2Cl2], tetrahedral
C) [Pt(NH3)2Cl2], square planar
D) [Co(NH3)5Cl]+, octahedral
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
41
The maximum number of unpaired electrons possible in any octahedral complex of a transition metal ion is:
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
42
Using the spectrochemical series (order of increasing D ) Cl - , the complex that exhibits the largest value of D is:
A) Ir(CN)63 -
B) IrCl63 -
C) Co(CN)64 -
D) Co(NH3)62+
E) Co(CN)63 -
A) Ir(CN)63 -
B) IrCl63 -
C) Co(CN)64 -
D) Co(NH3)62+
E) Co(CN)63 -

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
43
A titanium(III) complex (with a d1 electron configuration) absorbs light at 530 nm. What is D , the crystal field splitting in this complex, expressed in kJ/mol?
A) 1.25×10 - 27
B) 3.75×10 - 19
C) 226
D) 2.26×105
E) none of these
A) 1.25×10 - 27
B) 3.75×10 - 19
C) 226
D) 2.26×105
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
44
With different ligands a particular transition metal ion forms octahedral complexes with either zero or four unpaired electrons. How many d electrons are present in the metal?
A) 6
B) 7
C) 8
D) 4
E) 5
A) 6
B) 7
C) 8
D) 4
E) 5

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
45
The paramagnetism in complex ions of transition metals such as CoF63 - or NiCl42 - is a result of:
A) the charge on the ions.
B) electron pair donation by the ligands.
C) unpaired d-orbital electrons on the metal ions.
D) the square planar geometry possible with these metals.
E) paired d-orbital electrons on the metal ions.
A) the charge on the ions.
B) electron pair donation by the ligands.
C) unpaired d-orbital electrons on the metal ions.
D) the square planar geometry possible with these metals.
E) paired d-orbital electrons on the metal ions.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
46
Magnetic measurements on an octahedral complex of a transition metal show that it contains three unpaired electrons. Which of the following d-electron configurations of the metal ion are consistent with this fact?
Select the most complete answer.
A) d3
B) d3 and d7
C) d3, d5, and d7
D) d3, d5, d7, and d9
E) any d-electron configuration
Select the most complete answer.
A) d3
B) d3 and d7
C) d3, d5, and d7
D) d3, d5, d7, and d9
E) any d-electron configuration

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
47
Magnetic measurements on an octahedral complex of a transition metal ion show that it contains one unpaired electron. Which of the following d-electron configurations of the metal ion are consistent with this fact?
Select the most complete answer.
A) d1
B) d1 and d9
C) d5 and d7
D) d1, d5, d7, and d9
E) any d-electron configuration
Select the most complete answer.
A) d1
B) d1 and d9
C) d5 and d7
D) d1, d5, d7, and d9
E) any d-electron configuration

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
48
A titanium(III) complex (with a d1 electron configuration) absorbs light at 480 nm. What is D , the crystal field splitting in this complex, expressed in kJ/mol?
A) 1.38×10 - 27
B) 4.14×10 - 19
C) 250
D) 2.50×105
E) none of these
A) 1.38×10 - 27
B) 4.14×10 - 19
C) 250
D) 2.50×105
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
49
The species [RhCl6]3 - is considered a strong field, low spin complex. Which statement below is true regarding this complex?
A) The metal ion in this complex has a d3 electronic configuration with 1 unpaired electron.
B) The metal ion in this complex has a d3 electronic configuration witH ₃unpaired electrons.
C) The metal ion in this complex has a d6 electronic configuration with 0 unpaired electrons.
D) The metal ion in this complex has a d6 electronic configuration witH4 unpaired electrons.
E) The metal ion in this complex has a d9 electronic configuration with 1 unpaired electron.
A) The metal ion in this complex has a d3 electronic configuration with 1 unpaired electron.
B) The metal ion in this complex has a d3 electronic configuration witH ₃unpaired electrons.
C) The metal ion in this complex has a d6 electronic configuration with 0 unpaired electrons.
D) The metal ion in this complex has a d6 electronic configuration witH4 unpaired electrons.
E) The metal ion in this complex has a d9 electronic configuration with 1 unpaired electron.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
50
An octahedral complex of a d1 transition metal complex has D = 220 kJ/mol. At what wavelength does this complex absorb light?
A) 3.65×10 - 19 m
B) 5.51×1014 m
C) 1.81×10 - 15 m
D) 5.44×10 - 7 m
E) none of these
A) 3.65×10 - 19 m
B) 5.51×1014 m
C) 1.81×10 - 15 m
D) 5.44×10 - 7 m
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following complexes has the largest value of the crystal field splitting ( D )?
A) FeCl4 - (tetrahedral)
B) RuCl63 -
C) OsCl63 -
D) OsCl64 -
E) cannot be predicted
A) FeCl4 - (tetrahedral)
B) RuCl63 -
C) OsCl63 -
D) OsCl64 -
E) cannot be predicted

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
52
The species [Cr(CN)6]4 - is considered a strong field, low spin complex. Which statement below is true regarding this complex?
A) The metal ion in this complex has a d2 electronic configuration witH₂ unpaired electrons.
B) The metal ion in this complex has a d2 electronic configuration with 0 unpaired electrons.
C) The metal ion in this complex has a d4 electronic configuration witH₂ unpaired electrons.
D) The metal ion in this complex has a d4 electronic configuration witH4 unpaired electrons.
E) The metal ion in this complex has a d6 electronic configuration with 0 unpaired electrons.
A) The metal ion in this complex has a d2 electronic configuration witH₂ unpaired electrons.
B) The metal ion in this complex has a d2 electronic configuration with 0 unpaired electrons.
C) The metal ion in this complex has a d4 electronic configuration witH₂ unpaired electrons.
D) The metal ion in this complex has a d4 electronic configuration witH4 unpaired electrons.
E) The metal ion in this complex has a d6 electronic configuration with 0 unpaired electrons.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
53
Magnetic measurements on an octahedral complex of a transition metal ion show that it contains two unpaired electrons. Which of the following d-electron configurations of the metal ion are consistent with this fact?
Select the most complete answer.
A) d2
B) d2 and d8
C) d4 and d6
D) d2, d4, and d8
E) any d-electron configuration
Select the most complete answer.
A) d2
B) d2 and d8
C) d4 and d6
D) d2, d4, and d8
E) any d-electron configuration

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
54
With different ligands a particular transition metal ion forms octahedral complexes with either two or four unpaired electrons. How many d electrons are present in this metal ion?
A) 6
B) 2
C) 3
D) 4
E) 5
A) 6
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
55
With different ligands a particular transition metal ion forms octahedral complexes with either one or three unpaired electrons. How many d electrons are present in the metal?
A) 6
B) 7
C) 3
D) 4
E) 5
A) 6
B) 7
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
56
An octahedral complex of a d1 transition metal complex has D = 203 kJ/mol. At what wavelength does this complex absorb light?
A) 5.08×1014 m
B) 5.90×10 - 7 m
C) 1.97×10 - 15 m
D) 3.37×10 - 19 m
E) none of these
A) 5.08×1014 m
B) 5.90×10 - 7 m
C) 1.97×10 - 15 m
D) 3.37×10 - 19 m
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
57
The species [FeF6]3 - is considered a weak field, high spin complex. Which statement below is true regarding this complex?
A) The metal ion in this complex has a d3 electronic configuration witH ₃unpaired electrons.
B) The metal ion in this complex has a d3 electronic configuration with 1 unpaired electron.
C) The metal ion in this complex has a d5 electronic configuration witH5 unpaired electrons.
D) The metal ion in this complex has a d5 electronic configuration with 1 unpaired electron.
E) The metal ion in this complex has a d8 electronic configuration with 0 unpaired electrons.
A) The metal ion in this complex has a d3 electronic configuration witH ₃unpaired electrons.
B) The metal ion in this complex has a d3 electronic configuration with 1 unpaired electron.
C) The metal ion in this complex has a d5 electronic configuration witH5 unpaired electrons.
D) The metal ion in this complex has a d5 electronic configuration with 1 unpaired electron.
E) The metal ion in this complex has a d8 electronic configuration with 0 unpaired electrons.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
58
Magnetic measurements on an octahedral complex of a transition metal ion show that it contains four unpaired electrons. Which of the following d-electron configurations of the metal ion are consistent with this fact?
Select the most complete answer.
A) d4
B) d4 and d8
C) d4 and d6
D) d4, d6, and d8
E) any d-electron configuration
Select the most complete answer.
A) d4
B) d4 and d8
C) d4 and d6
D) d4, d6, and d8
E) any d-electron configuration

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
59
Using the spectrochemical series (order of increasing D ) Cl - , which metal complex absorbs light at the longest wavelength (lowest energy)?
A) Co(H2O)63+
B) Co(H2O)62+
C) Co(CN)63 -
D) Rh(NH3)62+
E) Rh(CN)62+
A) Co(H2O)63+
B) Co(H2O)62+
C) Co(CN)63 -
D) Rh(NH3)62+
E) Rh(CN)62+

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
60
With different ligands a particular transition metal ion forms octahedral complexes with either one or five unpaired electrons. How many d electrons are present in the metal?
A) 6
B) 7
C) 2
D) 4
E) 5
A) 6
B) 7
C) 2
D) 4
E) 5

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
61
Arrange the three complexes listed below in order of increasing crystal field energy , Δ . [Fe(H2O)6]3+ [Fe(H2O)6]2+ [Ru(H2O)6]3+
A) (smallest Δ ) [Fe(H2O)6]3+ < [Fe(H2O)6]2+ < [Ru(H2O)6]3+ (greatest Δ)
B) (smallest Δ ) [Fe(H2O)6]2+ < [Fe(H2O)6]3+ < [Ru(H2O)6]3+ (greatest Δ)
C) (smallest Δ ) [Ru(H2O)6]3+ < [Fe(H2O)6]3+ < [Fe(H2O)6]2+ (greatest Δ)
D) (smallest Δ ) [Ru(H2O)6]3+ < [Fe(H2O)6]2+ < [Fe(H2O)6]3+ (greatest Δ)
E) (smallest Δ ) [Fe(H2O)6]3+ < [Ru(H2O)6]3+ < [Fe(H2O)6]2+ (greatest Δ)
A) (smallest Δ ) [Fe(H2O)6]3+ < [Fe(H2O)6]2+ < [Ru(H2O)6]3+ (greatest Δ)
B) (smallest Δ ) [Fe(H2O)6]2+ < [Fe(H2O)6]3+ < [Ru(H2O)6]3+ (greatest Δ)
C) (smallest Δ ) [Ru(H2O)6]3+ < [Fe(H2O)6]3+ < [Fe(H2O)6]2+ (greatest Δ)
D) (smallest Δ ) [Ru(H2O)6]3+ < [Fe(H2O)6]2+ < [Fe(H2O)6]3+ (greatest Δ)
E) (smallest Δ ) [Fe(H2O)6]3+ < [Ru(H2O)6]3+ < [Fe(H2O)6]2+ (greatest Δ)

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
62
Which metal ion would have the largest value of D , when all are coordinated by the same ligands?
A) Re2+
B) Re3+
C) Tc3+
D) Mn2+
E) Mn3+
A) Re2+
B) Re3+
C) Tc3+
D) Mn2+
E) Mn3+

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following lists of metals all occur in nature as the free elements?
A) alkali metals
B) alkaline earth metals
C) Zn, Cd, Hg
D) Fe, Cr, Mn
E) Au, Pt, Ir
A) alkali metals
B) alkaline earth metals
C) Zn, Cd, Hg
D) Fe, Cr, Mn
E) Au, Pt, Ir

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
64
Most metallic elements occur in nature:
A) as the free element.
B) in a positive oxidation state.
C) in a negative oxidation state.
D) as liquids.
E) none of these
A) as the free element.
B) in a positive oxidation state.
C) in a negative oxidation state.
D) as liquids.
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
65
Consider the following statements:
I. D increases with increasing charge on a given transition metal.
II. For metal ions of the same charge, D increases down a group in the periodic table.
III. D is independent of the particular ligands in a complex. Which of these statements are true?
A) I only
B) II only
C) I and II
D) I and III
E) all of these
I. D increases with increasing charge on a given transition metal.
II. For metal ions of the same charge, D increases down a group in the periodic table.
III. D is independent of the particular ligands in a complex. Which of these statements are true?
A) I only
B) II only
C) I and II
D) I and III
E) all of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
66
Sulfide ores are roasted to:
A) separate the metal sulfide from other components.
B) remove volatile impurities from the ore.
C) convert the metal sulfides to metal oxides.
D) increase the oxidation state of the metal.
E) none of these.
A) separate the metal sulfide from other components.
B) remove volatile impurities from the ore.
C) convert the metal sulfides to metal oxides.
D) increase the oxidation state of the metal.
E) none of these.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
67
In the blast furnace, iron oxides are reduced by:
A) calcium carbonate.
B) carbon monoxide.
C) carbon dioxide.
D) all of these.
E) none of these.
A) calcium carbonate.
B) carbon monoxide.
C) carbon dioxide.
D) all of these.
E) none of these.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
68
In the oxygen furnace, impurities in iron are removed by:
A) oxidizing the non-metals such as C, Si and P.
B) vaporization of the impurities.
C) precipitation of impurities from aqueous solution.
D) fractional crystallization.
E) none of these.
A) oxidizing the non-metals such as C, Si and P.
B) vaporization of the impurities.
C) precipitation of impurities from aqueous solution.
D) fractional crystallization.
E) none of these.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
69
In the electrorefining process the impure metal is:
A) initially present in solution.
B) the cathode in an electrolysis cell.
C) the anode in an electrolysis cell.
D) preferentially reduced.
E) none of these
A) initially present in solution.
B) the cathode in an electrolysis cell.
C) the anode in an electrolysis cell.
D) preferentially reduced.
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
70
The concentration of ores by flotation takes advantage of differences in:
A) the densities of the components.
B) the chemical properties of the components.
C) the melting points of the components.
D) the solubilities of the components.
E) none of these.
A) the densities of the components.
B) the chemical properties of the components.
C) the melting points of the components.
D) the solubilities of the components.
E) none of these.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
71
Aluminum ores are treated with base to separate the aluminum from iron compounds. Which of the following chemical properties is important in this separation?
A) Aluminum is more easily oxidized than is iron.
B) The iron is reduced to the solid metal in basic solution.
C) Aluminum sulfide decomposes in basic solution, but iron sulfide does not.
D) Aluminum oxide is amphoteric and dissolves in base.
E) none of these
A) Aluminum is more easily oxidized than is iron.
B) The iron is reduced to the solid metal in basic solution.
C) Aluminum sulfide decomposes in basic solution, but iron sulfide does not.
D) Aluminum oxide is amphoteric and dissolves in base.
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following methods are used for the purification of metals?
I. distillation
II. zone refining
III. electrolysis
A) I and II only
B) II and III only
C) I and III only
D) I, II, and III
E) none of I, II, and III
I. distillation
II. zone refining
III. electrolysis
A) I and II only
B) II and III only
C) I and III only
D) I, II, and III
E) none of I, II, and III

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
73
When possible the preferred method of reduction of metals in ores is:
A) electrolysis.
B) displacement with a more reactive metal.
C) to use carbon as a reducing agent.
D) the formation of amalgams.
E) dependent on the impurities present in the ore.
A) electrolysis.
B) displacement with a more reactive metal.
C) to use carbon as a reducing agent.
D) the formation of amalgams.
E) dependent on the impurities present in the ore.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck


