Deck 18: Electrochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/112
Play
Full screen (f)
Deck 18: Electrochemistry
1
What is the oxidation state of vanadium in the VO2+ ion?
A) +2
B) +3
C) +4
D) +5
E) none of these
A) +2
B) +3
C) +4
D) +5
E) none of these
+4
2
The oxidation number of Cl in HClO3 is:
A) - 1
B) +6
C) +5
D) - 6
E) none of these
A) - 1
B) +6
C) +5
D) - 6
E) none of these
+5
3
What is the oxidation number for the carbon atom in COCl2?
A) 0
B) - 2
C) +2
D) +4
E) - 4
A) 0
B) - 2
C) +2
D) +4
E) - 4
+4
4
The oxidation number of Mn in MnO4 - is:
A) - 1
B) +4
C) +5
D) +7
E) none of these
A) - 1
B) +4
C) +5
D) +7
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
5
In the peroxydisulfate ion, S2O82 - , which has the Lewis structure shown below, what is the oxidation state of the sulfur?
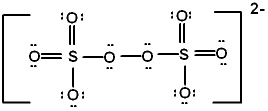
A) - 1
B) - 2
C) +6
D) +7
E) none of these
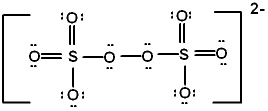
A) - 1
B) - 2
C) +6
D) +7
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
6
A compound has the empirical formula Na2O2. What is the oxidation state of the oxygen?
A) - 1
B) - 2
C) +1
D) +2
E) none of these
A) - 1
B) - 2
C) +1
D) +2
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
7
The oxidation number of Cl in HOCl is:
A) +1
B) +6
C) +3
D) - 1
E) none of these
A) +1
B) +6
C) +3
D) - 1
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
8
The oxidation state of Cr in Cr2O72 - is:
A) +12
B) +6
C) +7
D) +4
E) none of these
A) +12
B) +6
C) +7
D) +4
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
9
What is the oxidation number of the chromium atom in the compound, BaCr2O7?
A) 0
B) +2
C) +6
D) +7
E) +12
A) 0
B) +2
C) +6
D) +7
E) +12

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
10
The "super-ion battery" contains K2FeO4 as a replacement for MnO2 in alkaline dry batteries. What is the charge of the iron atom in the compound K2FeO4?
A) +2
B) +4
C) +6
D) 0
E) - 4
A) +2
B) +4
C) +6
D) 0
E) - 4

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
11
The oxidation state of S in SO2 is:
A) +2
B) +4
C) - 2
D) - 4
E) none of these
A) +2
B) +4
C) - 2
D) - 4
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
12
What is the oxidation number of the Cr in H2Cr2O7?
A) +5
B) +3
C) +6
D) +4
E) none of these
A) +5
B) +3
C) +6
D) +4
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
13
In the azide ion, which has the Lewis structure shown below, the oxidation state of the central N atom is:
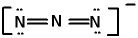
A) +1
B) +1/3
C) - 1/3
D) - 1
E) none of these
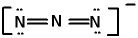
A) +1
B) +1/3
C) - 1/3
D) - 1
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
14
What is the oxidation number of the manganese atom in the formula for the compound KMnO4?
A) - 1
B) +1
C) +3
D) +5
E) +7
A) - 1
B) +1
C) +3
D) +5
E) +7

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
15
The oxidation state of sulfur in SO3 is:
A) - 3
B) 0
C) +3
D) +6
E) none of these
A) - 3
B) 0
C) +3
D) +6
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
16
What is the oxidation number of the carbon atom in the compound CO?
A) - 4
B) - 2
C) 0
D) +2
E) +4
A) - 4
B) - 2
C) 0
D) +2
E) +4

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
17
In which of the following compounds is the oxidation number of the nitrogen atom equal to +5?
A) NH3
B) N2O4
C) NO
D) HNO3
E) NO2
A) NH3
B) N2O4
C) NO
D) HNO3
E) NO2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
18
The oxidation state of chlorine in ClO4 - is:
A) - 1
B) +4
C) +5
D) +7
E) none of these
A) - 1
B) +4
C) +5
D) +7
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
19
What is the oxidation number of the chlorine in the compound, Ba(ClO2)2?
A) 0
B) - 1
C) - 2
D) +3
E) +6
A) 0
B) - 1
C) - 2
D) +3
E) +6

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
20
The oxidation state of oxygen in O2F2 is:
A) - 1
B) - 2
C) +2
D) +1
E) none of these
A) - 1
B) - 2
C) +2
D) +1
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
21
In the properly balanced half-reaction (acid solution) for the system H3AsO3→H2AsO4 - , what else appears on the right side besides H2AsO4 - ?
A) 2 H+ + 2 e -
B) 3 H+ + 2 e -
C) 2 H2O + 2 e -
D) 2 H+ + 3 e -
E) H+
A) 2 H+ + 2 e -
B) 3 H+ + 2 e -
C) 2 H2O + 2 e -
D) 2 H+ + 3 e -
E) H+

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
22
Start with the skeleton half-reaction CrO2 - →CrO42 - . When balanced in basic solution, what other species appear on the right side of the equation, in addition to CrO42 - ?
A) 2 H2O + 3 e -
B) 4 H+ + 3 e -
C) 2 H2O
D) 4 OH -
E) none of these
A) 2 H2O + 3 e -
B) 4 H+ + 3 e -
C) 2 H2O
D) 4 OH -
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
23
After balancing the following reaction under acidic conditions , what is the coefficient for the species, BiO31 - ?
Mn2+ (aq) + BiO31 - (aq)→Bi3+ (aq) + MnO41 - (aq) [Acidic conditions]
A) 1
B) 2
C) 3
D) 5
E) 6
Mn2+ (aq) + BiO31 - (aq)→Bi3+ (aq) + MnO41 - (aq) [Acidic conditions]
A) 1
B) 2
C) 3
D) 5
E) 6

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
24
What is the oxidizing agent in the following reaction?
H2O + 2 MnO41 - (aq) + I - (aq)→2 MnO2 (s) + IO31 - (aq) + 2 OH - (aq)
A) H2O
B) MnO41 -
C) I -
D) MnO2
E) IO31 -
H2O + 2 MnO41 - (aq) + I - (aq)→2 MnO2 (s) + IO31 - (aq) + 2 OH - (aq)
A) H2O
B) MnO41 -
C) I -
D) MnO2
E) IO31 -

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
25
In the redox reaction:
3 S2 - + 2 MnO4 - + 8 H2O→3 S + 2 MnO2 + 4 H2O + 8 OH - the S2 - changes oxidation state to give the element S. Which statement below is true?
A) S2 - has been oxidized.
B) S2 - has been reduced.
C) S2 - is an oxidizing agent.
D) S2 - gains electrons.
E) more than one of these
3 S2 - + 2 MnO4 - + 8 H2O→3 S + 2 MnO2 + 4 H2O + 8 OH - the S2 - changes oxidation state to give the element S. Which statement below is true?
A) S2 - has been oxidized.
B) S2 - has been reduced.
C) S2 - is an oxidizing agent.
D) S2 - gains electrons.
E) more than one of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
26
The redox reaction (unbalanced), Cl2→ClO3 - + Cl - , occurs in alkaline solution. The number of electrons transferred in the balanced equation is:
A) 2
B) 4
C) 6
D) 8
E) 5
A) 2
B) 4
C) 6
D) 8
E) 5

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
27
After balancing the following reaction under basic conditions , how many mole equivalents of water are required and on which side of the reaction do they appear?
CrO42 - (aq) + Cu (s)→Cr(OH)3 (s) + Cu(OH)2 (s) [Basic conditions]
A) 2 moles of H2O on the reactant side
B) 2 moles of H2O on the product side
C) 4 moles of H2O on the product side
D) 4 moles of H2O on the reactant side
E) 8 moles of H2O on the reactant side
CrO42 - (aq) + Cu (s)→Cr(OH)3 (s) + Cu(OH)2 (s) [Basic conditions]
A) 2 moles of H2O on the reactant side
B) 2 moles of H2O on the product side
C) 4 moles of H2O on the product side
D) 4 moles of H2O on the reactant side
E) 8 moles of H2O on the reactant side

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
28
After balancing the following reaction under acidic conditions , how many mole equivalents of water are required and on which side of the reaction do they appear?
MnO41 - (aq) + Cl1 - (aq)→Mn2+ (aq) + Cl2 (g) [Acidic conditions]
A) 2 moles of H2O on the reactant side
B) 2 moles of H2O on the product side
C) 4 moles of H2O on the product side
D) 8 moles of H2O on the product side
E) 10 moles of H2O on the reactant side
MnO41 - (aq) + Cl1 - (aq)→Mn2+ (aq) + Cl2 (g) [Acidic conditions]
A) 2 moles of H2O on the reactant side
B) 2 moles of H2O on the product side
C) 4 moles of H2O on the product side
D) 8 moles of H2O on the product side
E) 10 moles of H2O on the reactant side

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
29
In the oxidation-reduction reaction shown below, the number of electrons transferred is:
H2O + 3 ClO - (aq)→ClO2 - (aq) + Cl2 (aq) + 2 OH - (aq)
A) 1
B) 2
C) 3
D) 4
E) 5
H2O + 3 ClO - (aq)→ClO2 - (aq) + Cl2 (aq) + 2 OH - (aq)
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
30
After balancing the following reaction under acidic conditions , what is the coefficient for the species, Cl1 - ?
MnO41 - (aq) + Cl1 - (aq)→Mn2+ (aq) + Cl2 (g) [Acidic conditions]
A) 1
B) 2
C) 5
D) 7
E) 10
MnO41 - (aq) + Cl1 - (aq)→Mn2+ (aq) + Cl2 (g) [Acidic conditions]
A) 1
B) 2
C) 5
D) 7
E) 10

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
31
After balancing the following reaction under basic conditions , what is the coefficient for the copper?
CrO42 - (aq) + Cu (s)→Cr(OH)3 (s) + Cu(OH)2 (s) [Basic conditions]
A) 1
B) 2
C) 3
D) 6
E) 9
CrO42 - (aq) + Cu (s)→Cr(OH)3 (s) + Cu(OH)2 (s) [Basic conditions]
A) 1
B) 2
C) 3
D) 6
E) 9

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
32
Which equation listed below is correct after balancing the following unbalanced equation under basic conditions ?
MnO41 - (aq) + Br1 - (aq)→MnO2 (s) + BrO31 - (aq) [Basic conditions]
A) MnO41 - (aq) + Br1 - (aq) + 2 OH - (aq)→ MnO2 (s) + BrO31 - (aq) + H2O
B) MnO41 - (aq) + 2 Br1 - (aq) + 8 OH - (aq)→ MnO2 (s) + 2 BrO31 - (aq) + 4 H2O
C) 2 MnO41 - (aq) + Br1 - (aq) + H2O→ 2 MnO2 (s) + BrO31 - (aq) + 2 OH - (aq)
D) 2 H+ (aq) + 2 MnO41 - (aq) + Br1 - (aq)→ 2 MnO2 (s) + BrO31 - (aq) + H2O
E) 2 MnO41 - (aq) + 2 Br1 - (aq) + 4 OH - (aq)→ 2 MnO2 (s) + 2 BrO31 - (aq) + 2 H2O
MnO41 - (aq) + Br1 - (aq)→MnO2 (s) + BrO31 - (aq) [Basic conditions]
A) MnO41 - (aq) + Br1 - (aq) + 2 OH - (aq)→ MnO2 (s) + BrO31 - (aq) + H2O
B) MnO41 - (aq) + 2 Br1 - (aq) + 8 OH - (aq)→ MnO2 (s) + 2 BrO31 - (aq) + 4 H2O
C) 2 MnO41 - (aq) + Br1 - (aq) + H2O→ 2 MnO2 (s) + BrO31 - (aq) + 2 OH - (aq)
D) 2 H+ (aq) + 2 MnO41 - (aq) + Br1 - (aq)→ 2 MnO2 (s) + BrO31 - (aq) + H2O
E) 2 MnO41 - (aq) + 2 Br1 - (aq) + 4 OH - (aq)→ 2 MnO2 (s) + 2 BrO31 - (aq) + 2 H2O

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
33
After balancing the following reaction under basic conditions , how many electrons are transferred?
MnO41 - (aq) + Br1 - (aq)→MnO2 (s) + BrO31 - (aq) [Basic conditions]
A) 1 electron
B) 2 electrons
C) 3 electrons
D) 6 electrons
E) 9 electrons
MnO41 - (aq) + Br1 - (aq)→MnO2 (s) + BrO31 - (aq) [Basic conditions]
A) 1 electron
B) 2 electrons
C) 3 electrons
D) 6 electrons
E) 9 electrons

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
34
What species becomes oxidized and what species behaves as the reducing agent in the following oxidation-reduction reaction?
2 H₂ O ( ) + Al (s) + MnO4 1 - (aq) Al(OH) 4 1 - (aq) + MnO₂ (s)
) + Al (s) + MnO4 1 - (aq) Al(OH) 4 1 - (aq) + MnO₂ (s)
A) Al becomes oxidized and Al behaves as the reducing agent.
B) Al becomes oxidized and MnO41 - behaves as the reducing agent.
C) MnO41 - becomes oxidized and MnO41 - behaves as the reducing agent.
D) MnO41 - becomes oxidized and Al behaves as the reducing agent.
E) MnO41 - becomes oxidized and H2O behaves as the reducing agent.
2 H₂ O (
 ) + Al (s) + MnO4 1 - (aq) Al(OH) 4 1 - (aq) + MnO₂ (s)
) + Al (s) + MnO4 1 - (aq) Al(OH) 4 1 - (aq) + MnO₂ (s)A) Al becomes oxidized and Al behaves as the reducing agent.
B) Al becomes oxidized and MnO41 - behaves as the reducing agent.
C) MnO41 - becomes oxidized and MnO41 - behaves as the reducing agent.
D) MnO41 - becomes oxidized and Al behaves as the reducing agent.
E) MnO41 - becomes oxidized and H2O behaves as the reducing agent.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
35
After balancing the following reaction under acidic conditions , how many electrons are transferred?
Mn2+ (aq) + BiO31 - (aq)→Bi3+ (aq) + MnO41 - (aq) [Acidic conditions]
A) 1 electron
B) 2 electrons
C) 5 electrons
D) 7 electrons
E) 10 electrons
Mn2+ (aq) + BiO31 - (aq)→Bi3+ (aq) + MnO41 - (aq) [Acidic conditions]
A) 1 electron
B) 2 electrons
C) 5 electrons
D) 7 electrons
E) 10 electrons

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
36
In the following balanced redox reaction:
14 HCl + K2Cr2O7 + 6 FeCl2→2 CrCl3 + 6 FeCl3 + 2 KCl + 7 H 2O
A) K2Cr2O7 is oxidized.
B) FeCl2 is a reducing agent.
C) HCl is an oxidizing agent.
D) HCl is oxidized.
E) none of these.
14 HCl + K2Cr2O7 + 6 FeCl2→2 CrCl3 + 6 FeCl3 + 2 KCl + 7 H 2O
A) K2Cr2O7 is oxidized.
B) FeCl2 is a reducing agent.
C) HCl is an oxidizing agent.
D) HCl is oxidized.
E) none of these.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
37
Start with the skeleton half-reaction NO3 - →NO2. When balanced in acid solution, what other species appear on the left side of the equation, in addition to NO3 -?
A) H2O + 2 e -
B) 2 H+ + e -
C) H+ + 2 e -
D) 2 H+
E) none of these
A) H2O + 2 e -
B) 2 H+ + e -
C) H+ + 2 e -
D) 2 H+
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
38
Given the skeleton half-reaction:
Na2C2O4→CO2 + Na+ When the half-reaction is properly balanced we find that the half-reaction represents:
A) an oxidation.
B) a reduction.
C) neither
Na2C2O4→CO2 + Na+ When the half-reaction is properly balanced we find that the half-reaction represents:
A) an oxidation.
B) a reduction.
C) neither

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
39
Start with the skeleton half-reaction NO2 - →NO3 - . When balanced in basic solution, what other species appear on the right side of the equation, in addition to NO3 - ?
A) 2 H+ + 2 e -
B) 2 OH -
C) H2O + 2 e -
D) H2O
E) none of these
A) 2 H+ + 2 e -
B) 2 OH -
C) H2O + 2 e -
D) H2O
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
40
In the oxidation-reduction equation (balanced):
H2O + 3 ClO - (aq)→ClO2 - (aq) + Cl2 (aq) + 2 OH - (aq)
A) ClO - is reduced.
B) ClO - is oxidized.
C) ClO - is an oxidizing agent.
D) ClO - is a reducing agent.
E) choices a-d are all correct statements.
H2O + 3 ClO - (aq)→ClO2 - (aq) + Cl2 (aq) + 2 OH - (aq)
A) ClO - is reduced.
B) ClO - is oxidized.
C) ClO - is an oxidizing agent.
D) ClO - is a reducing agent.
E) choices a-d are all correct statements.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following voltaic cell reactions would not require a salt bridge?
A) Ce4+ + Fe2+→ Ce3+ + Fe3+
B) Cu (s) + 2 Ag+→ Cu2+ + 2 Ag (s)
C) H2 (g) + 2 AgCl (s)→ 2 H+ + 2 Cl -
D) 2 Cu2+ + 4 I - → CuI (s) + I2 (s)
E) all of these cells require a salt bridge
A) Ce4+ + Fe2+→ Ce3+ + Fe3+
B) Cu (s) + 2 Ag+→ Cu2+ + 2 Ag (s)
C) H2 (g) + 2 AgCl (s)→ 2 H+ + 2 Cl -
D) 2 Cu2+ + 4 I - → CuI (s) + I2 (s)
E) all of these cells require a salt bridge

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
42
Exhibit 18-1 Consider the figure of a generic voltaic cell below to answer the following question(s). 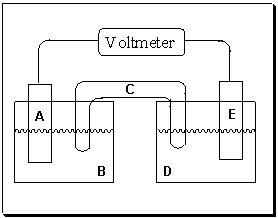
Refer to Exhibit 18-1. If electrons are flowing in the direction from half-cell D to half-cell B, which component in this voltaic cell represents the electrode where oxidation occurs ?
A) A
B) B
C) C
D) D
E) E

Refer to Exhibit 18-1. If electrons are flowing in the direction from half-cell D to half-cell B, which component in this voltaic cell represents the electrode where oxidation occurs ?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
43
A sample solution contains Sn2+ ions and is titrated with a 0.0500 M solution of Ce4+, consuming 35.44 mL of the Ce4+ solution. The products of the redox reaction are Sn4+ and Ce3+. What mass of Sn2+ was present in the original solution?
A) 8.86×10 - 4 g
B) 0.105 g
C) 0.210 g
D) 0.420 g
E) none of these
A) 8.86×10 - 4 g
B) 0.105 g
C) 0.210 g
D) 0.420 g
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
44
A sample containing Fe2O3 is dissolved and the Fe3+ is reduced to Fe2+, which is titrated with standard 0.0200 M KMnO4. The skeleton equation is:
Fe2+ + MnO4 - →Fe3+ + Mn2+ How many grams of iron (molar mass = 55.85 g/mol) are in the sample, if 25.0 mL of KMnO4 are required in the titration?
A) 0.140 g
B) 0.0279 g
C) 5.58×10 - 3 g
D) 140 g
E) need more information
Fe2+ + MnO4 - →Fe3+ + Mn2+ How many grams of iron (molar mass = 55.85 g/mol) are in the sample, if 25.0 mL of KMnO4 are required in the titration?
A) 0.140 g
B) 0.0279 g
C) 5.58×10 - 3 g
D) 140 g
E) need more information

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
45
Exhibit 18-1 Consider the figure of a generic voltaic cell below to answer the following question(s). 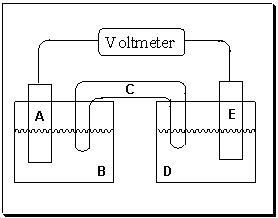
Refer to Exhibit 18-1. If electrons are flowing in the direction from half-cell D to half-cell B, what is the purpose of component C in the voltaic cell above?
A) It is a salt solution that provides cations that can migrate to compartment B and anions that migrate to compartment D in an effort to balance the charge as electricity flows.
B) It is a salt solution that provides anions that can migrate to compartment B and cations that migrate to compartment D in an effort to balance the charge as electricity flows.
C) It allows mixing of solutions in compartments B and D.
D) Its purpose is to hold the two cells together.
E) Electricity flows through this region to complete the circuit as electricity flows.

Refer to Exhibit 18-1. If electrons are flowing in the direction from half-cell D to half-cell B, what is the purpose of component C in the voltaic cell above?
A) It is a salt solution that provides cations that can migrate to compartment B and anions that migrate to compartment D in an effort to balance the charge as electricity flows.
B) It is a salt solution that provides anions that can migrate to compartment B and cations that migrate to compartment D in an effort to balance the charge as electricity flows.
C) It allows mixing of solutions in compartments B and D.
D) Its purpose is to hold the two cells together.
E) Electricity flows through this region to complete the circuit as electricity flows.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following half-reactions could occur at the negative electrode of a voltaic cell?
A) Cu2+ + 2 e - → Cu
B) Fe3+ + e - → Fe2+
C) Zn→ Zn2+ + 2 e -
D) Cu→ Cu2+ + 2 e -
E) both answers c and d
A) Cu2+ + 2 e - → Cu
B) Fe3+ + e - → Fe2+
C) Zn→ Zn2+ + 2 e -
D) Cu→ Cu2+ + 2 e -
E) both answers c and d

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
47
In a voltaic cell:
A) oxidation occurs at the positive electrode.
B) electrons flow through the external circuit from the negative to the positive electrode.
C) the charge is carried through the solution by electrons and protons.
D) electrical energy is consumed to cause a chemical change.
E) none of these.
A) oxidation occurs at the positive electrode.
B) electrons flow through the external circuit from the negative to the positive electrode.
C) the charge is carried through the solution by electrons and protons.
D) electrical energy is consumed to cause a chemical change.
E) none of these.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
48
After balancing the following reaction under acidic conditions , how many mole equivalents of water are required and on which side of the reaction do they appear?
CrO42 - (aq) + N2O (aq)→Cr3+ (aq) + NO (g) [Acidic conditions]
A) 2 moles of H2O on the reactant side
B) 2 moles of H2O on the product side
C) 5 moles of H2O on the product side
D) 5 moles of H2O on the reactant side
E) 10 moles of H2O on the reactant side
CrO42 - (aq) + N2O (aq)→Cr3+ (aq) + NO (g) [Acidic conditions]
A) 2 moles of H2O on the reactant side
B) 2 moles of H2O on the product side
C) 5 moles of H2O on the product side
D) 5 moles of H2O on the reactant side
E) 10 moles of H2O on the reactant side

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
49
Exhibit 18-1 Consider the figure of a generic voltaic cell below to answer the following question(s). 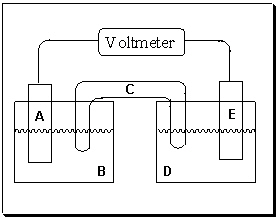
Refer to Exhibit 18-1. If component A is the positive electrode and component E is the negative electrode, which statement is true?
A) Electrons are flowing from left to right (A→ E) in this figure. Electrode E is "swelling" as metal ions plate onto its surface.
B) Electrons are flowing from left to right (A→ E) in this figure. Electrode E is corroding as metal atoms dissolve into solution D.
C) Electrons are flowing from right to left (E→ A) in this figure. Electrode E is "swelling" as metal ions plate onto its surface.
D) Electrons are flowing from right to left (E→ A) in this figure. Electrode E is corroding as metal atoms dissolve into solution D.
E) Electrons are not flowing in this voltaic cell unless it is provided by an outside source.

Refer to Exhibit 18-1. If component A is the positive electrode and component E is the negative electrode, which statement is true?
A) Electrons are flowing from left to right (A→ E) in this figure. Electrode E is "swelling" as metal ions plate onto its surface.
B) Electrons are flowing from left to right (A→ E) in this figure. Electrode E is corroding as metal atoms dissolve into solution D.
C) Electrons are flowing from right to left (E→ A) in this figure. Electrode E is "swelling" as metal ions plate onto its surface.
D) Electrons are flowing from right to left (E→ A) in this figure. Electrode E is corroding as metal atoms dissolve into solution D.
E) Electrons are not flowing in this voltaic cell unless it is provided by an outside source.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
50
After balancing the following reaction under basic conditions , what is the coefficient for the sulfite species, SO32 - ?
SO32 - (aq) + Cl2 (g)→SO42 - (aq) + Cl1 - (aq) [Basic conditions]
A) 1
B) 2
C) 3
D) 4
E) 6
SO32 - (aq) + Cl2 (g)→SO42 - (aq) + Cl1 - (aq) [Basic conditions]
A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
51
Consider a voltaic cell that operates from the following redox reaction:
Al (s) + Cr3+ (aq)→Al3+ (aq) + Cr (s) What half-reaction operates at the positive electrode of this cell?
A) Al (s)→ Al3+ (aq) + 3 e -
B) Al3+ (aq) + 3 e - → Al (s)
C) Cr (s)→ Cr3+ (aq) + 3 e -
D) Cr3+ (aq) + 3 e - → Cr (s)
E) Al3+ (aq) + 3 e - → Cr (s)
Al (s) + Cr3+ (aq)→Al3+ (aq) + Cr (s) What half-reaction operates at the positive electrode of this cell?
A) Al (s)→ Al3+ (aq) + 3 e -
B) Al3+ (aq) + 3 e - → Al (s)
C) Cr (s)→ Cr3+ (aq) + 3 e -
D) Cr3+ (aq) + 3 e - → Cr (s)
E) Al3+ (aq) + 3 e - → Cr (s)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
52
After balancing the following reaction under acidic conditions , what is the coefficient for N2O?
CrO42 - (aq) + N2O (aq)→Cr3+ (aq) + NO (g) [Acidic conditions]
A) 1
B) 2
C) 3
D) 4
E) 6
CrO42 - (aq) + N2O (aq)→Cr3+ (aq) + NO (g) [Acidic conditions]
A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
53
A 30.0 mL sample of solution containing Fe2+ requires 25.0 mL of 0.0200 M KMnO4 solution to react completely. The products of the reaction are Fe3+ and Mn2+. What is the concentration of Fe2+ in the original solution?
A) 3.33×10 - 3 M
B) 1.67×10 - 2 M
C) 8.33×10 - 2 M
D) 0.500 M
E) none of these
A) 3.33×10 - 3 M
B) 1.67×10 - 2 M
C) 8.33×10 - 2 M
D) 0.500 M
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
54
After balancing the following reaction under basic conditions , how many mole equivalents of hydroxide are required and on which side of the reaction do they appear?
SO32 - (aq) + Cl2 (g)→SO42 - (aq) + Cl1 - (aq) [Basic conditions]
A) 2 moles of OH - on the reactant side
B) 2 moles of OH - on the product side
C) 5 moles of OH - on the product side
D) 5 moles of OH - on the reactant side
E) 10 moles of OH - on the reactant side
SO32 - (aq) + Cl2 (g)→SO42 - (aq) + Cl1 - (aq) [Basic conditions]
A) 2 moles of OH - on the reactant side
B) 2 moles of OH - on the product side
C) 5 moles of OH - on the product side
D) 5 moles of OH - on the reactant side
E) 10 moles of OH - on the reactant side

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
55
With respect to any voltaic cell, at what electrode does reduction occur and what is the sign convention given to this electrode?
A) anode; "+" terminal
B) anode; " - " terminal
C) cathode; "+" terminal
D) cathode; " - " terminal
E) None of these
A) anode; "+" terminal
B) anode; " - " terminal
C) cathode; "+" terminal
D) cathode; " - " terminal
E) None of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following processes could occur at the positive electrode of a voltaic cell?
A) Cu2+ + 2 e - → Cu
B) Fe3+ + e - → Fe2+
C) Zn→ Zn2+ + 2 e -
D) Cu→ Cu2+ + 2 e -
E) both answers a and b
A) Cu2+ + 2 e - → Cu
B) Fe3+ + e - → Fe2+
C) Zn→ Zn2+ + 2 e -
D) Cu→ Cu2+ + 2 e -
E) both answers a and b

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
57
Consider a voltaic cell that operates from the following redox reaction:
Mn (s) + Ag1+ (aq)→Mn2+ (aq) + Ag (s) What half-reaction operates at the negative electrode of this cell?
A) Mn (s)→ Mn2+ (aq) + 2 e -
B) Ag1+ (aq) + 1 e - → Ag (s)
C) Ag (s)→ Ag1+ (aq) + 1 e -
D) Mn2+ (aq) + 2 e - → Mn (s)
E) Mn2+ (aq) + 2 e - → Ag (s)
Mn (s) + Ag1+ (aq)→Mn2+ (aq) + Ag (s) What half-reaction operates at the negative electrode of this cell?
A) Mn (s)→ Mn2+ (aq) + 2 e -
B) Ag1+ (aq) + 1 e - → Ag (s)
C) Ag (s)→ Ag1+ (aq) + 1 e -
D) Mn2+ (aq) + 2 e - → Mn (s)
E) Mn2+ (aq) + 2 e - → Ag (s)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
58
A voltaic cell consists of two half-cells:
Zinc metal immersed in a 1 M solution of ZnCl2 and copper immersed in a 1M solution of CuSO4. The copper electrode is positive. What is the spontaneous chemical reaction that occurs in this cell?
A) Cu (s) + Zn2+→ Zn (s) + Cu2+
B) Zn (s) + Cu2+→ Zn2+ + Cu (s)
C) Zn2+ + Cu2+→ Zn (s) + Cu (s)
D) No chemical reaction occurs.
E) none of these
Zinc metal immersed in a 1 M solution of ZnCl2 and copper immersed in a 1M solution of CuSO4. The copper electrode is positive. What is the spontaneous chemical reaction that occurs in this cell?
A) Cu (s) + Zn2+→ Zn (s) + Cu2+
B) Zn (s) + Cu2+→ Zn2+ + Cu (s)
C) Zn2+ + Cu2+→ Zn (s) + Cu (s)
D) No chemical reaction occurs.
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
59
Under standard conditions, the spontaneous reaction in a voltaic cell i S2U3+ + Cd2+→2 U4+ + Cd (s). Consider the three statements below and choose the correct answer.
I. The cadmium is the positive electrode in the cell.
II. A salt bridge is necessary in this cell.
III. The U3+ is oxidized in the cell.
A) only I is true
B) only II is true
C) only III is true
D) I, II, and III are all true
E) only 2 of the three statements are true
I. The cadmium is the positive electrode in the cell.
II. A salt bridge is necessary in this cell.
III. The U3+ is oxidized in the cell.
A) only I is true
B) only II is true
C) only III is true
D) I, II, and III are all true
E) only 2 of the three statements are true

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
60
In a voltaic cell, the spontaneous reaction that occurs is Cr (s) + Cu2+→Cu (s) + Cr2+. Consider the following three statements and choose the correct answer.
I. The chromium metal is the negative electrode.
II. A salt bridge is unnecessary in this voltaic cell.
III. Copper (II) ions are reduced.
A) only I is true
B) only III is true
C) II and III are true, I is false
D) I and III are true, II is false
E) I, II, and III are all true
I. The chromium metal is the negative electrode.
II. A salt bridge is unnecessary in this voltaic cell.
III. Copper (II) ions are reduced.
A) only I is true
B) only III is true
C) II and III are true, I is false
D) I and III are true, II is false
E) I, II, and III are all true

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
61
Exhibit 18-2 Consider a voltaic cell that is constructed from a strip of Ag dipped in an AgNO3 solution and a Fe (s) strip dipped in a solution of FeCl2 to answer the following question(s). An external circuit and a salt bridge connect these two electrodes.
Refer to Exhibit 18-2. Which electrode is the negative electrode and what is the standard potential E ° cell for this voltaic cell?
A) The Fe2+/Fe couple is the negative electrode and E ° cell = 1.24 V.
B) The Fe2+/Fe couple is the negative electrode and E ° cell = 0.36 V.
C) The Fe2+/Fe couple is the negative electrode and E ° cell = - 1.24 V.
D) The Ag+/Ag couple is the negative electrode and E ° cell = 1.24 V.
E) The Ag+/Ag couple is the negative electrode and E ° cell = 0.36 V.
Refer to Exhibit 18-2. Which electrode is the negative electrode and what is the standard potential E ° cell for this voltaic cell?
A) The Fe2+/Fe couple is the negative electrode and E ° cell = 1.24 V.
B) The Fe2+/Fe couple is the negative electrode and E ° cell = 0.36 V.
C) The Fe2+/Fe couple is the negative electrode and E ° cell = - 1.24 V.
D) The Ag+/Ag couple is the negative electrode and E ° cell = 1.24 V.
E) The Ag+/Ag couple is the negative electrode and E ° cell = 0.36 V.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
62
Given:
Cu (s) + 2 Ag+→2 Ag (s) + Cu2+ E ° = +0.46 V Cu2+ + H2 (g)→Cu (s) + 2 H+ E ° = +0.34 V Find the standard potential for the cell reaction for:
2 Ag+ + H2 (g)→2 Ag + 2 H+
A) +0.80 V
B) +0.40 V
C) +0.12 V
D) - 0.12 V
E) none of these
Cu (s) + 2 Ag+→2 Ag (s) + Cu2+ E ° = +0.46 V Cu2+ + H2 (g)→Cu (s) + 2 H+ E ° = +0.34 V Find the standard potential for the cell reaction for:
2 Ag+ + H2 (g)→2 Ag + 2 H+
A) +0.80 V
B) +0.40 V
C) +0.12 V
D) - 0.12 V
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
63
Exhibit 18-3 Use the standard reduction potentials below to answer the following question(s).
Ce4+(aq) + e - →Ce3+(aq) E ° = 1.61 V
Fe3+(aq) + e - →Fe2+(aq) E ° = 0.77 V
I2(s) + 2 e - →2 I - (aq) E ° = 0.54 V
2 H+(aq) + 2 e - →H2(g) E ° = 0.000 V
Cd2+(aq) + 2 e - →Cd(s) E ° = - 0.40 V
U4+(aq) + e - →U3+(aq) E ° = - 0.61 V
CdCO3(s) + 2 e - →Cd(s) + CO32 - (aq) E ° = - 0.74 V
Zn2+(aq) + 2 e - →Zn(s) E ° = - 0.76 V
U3+(aq) + 3 e - →U(s) E ° = - 1.80 V
Mg2+(aq) + 2 e - →Mg(s) E ° = - 2.37 V
Refer to Exhibit 18-3. Find the standard potential for the reaction:
2 U4+ + 2 I - →2 U3+ + I2 (s)
A) +0.58 V
B) - 0.04 V
C) - 0.07 V
D) - 1.15 V
E) none of these
Ce4+(aq) + e - →Ce3+(aq) E ° = 1.61 V
Fe3+(aq) + e - →Fe2+(aq) E ° = 0.77 V
I2(s) + 2 e - →2 I - (aq) E ° = 0.54 V
2 H+(aq) + 2 e - →H2(g) E ° = 0.000 V
Cd2+(aq) + 2 e - →Cd(s) E ° = - 0.40 V
U4+(aq) + e - →U3+(aq) E ° = - 0.61 V
CdCO3(s) + 2 e - →Cd(s) + CO32 - (aq) E ° = - 0.74 V
Zn2+(aq) + 2 e - →Zn(s) E ° = - 0.76 V
U3+(aq) + 3 e - →U(s) E ° = - 1.80 V
Mg2+(aq) + 2 e - →Mg(s) E ° = - 2.37 V
Refer to Exhibit 18-3. Find the standard potential for the reaction:
2 U4+ + 2 I - →2 U3+ + I2 (s)
A) +0.58 V
B) - 0.04 V
C) - 0.07 V
D) - 1.15 V
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
64
Consider a voltaic cell that operates from the following redox reaction:
Mn (s) + Ag1+ (aq)→Mn2+ (aq) + Ag (s) What is the value of E ° cell if E ° red(Ag1+/Ag) = 0.80 V and E ° red(Mn2+/Mn) = - 1.18 V?
A) E ° cell = - 1.98 V
B) E ° cell = - 0.94 V
C) E ° cell = - 0.38 V
D) E ° cell = 0.38 V
E) E ° cell = 1.98 V
Mn (s) + Ag1+ (aq)→Mn2+ (aq) + Ag (s) What is the value of E ° cell if E ° red(Ag1+/Ag) = 0.80 V and E ° red(Mn2+/Mn) = - 1.18 V?
A) E ° cell = - 1.98 V
B) E ° cell = - 0.94 V
C) E ° cell = - 0.38 V
D) E ° cell = 0.38 V
E) E ° cell = 1.98 V

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
65
Given:
Zn (s) + Sn2+→Sn (s) + Zn2+ with E ° = +0.62 V What is the equilibrium constant for this reaction at 298 K?
A) 21
B) 1×1021
C) 5×1020
D) 3×1010
E) 4×10 - 31
Zn (s) + Sn2+→Sn (s) + Zn2+ with E ° = +0.62 V What is the equilibrium constant for this reaction at 298 K?
A) 21
B) 1×1021
C) 5×1020
D) 3×1010
E) 4×10 - 31

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
66
Consider the following redox reaction:
2 Li+ (aq) + Fe (s)→Fe2+ (aq) + 2 Li (s) Which statement below is true regarding (1) the spontaneity of this reaction and (2) the value of E ° cell?
E ° red(Li1+/Li) = - 3.05V and E ° red(Fe2+/Fe) = - 0.44 V
A) This reaction is spontaneous as written and E ° cell = - 2.61 V.
B) This reaction is non-spontaneous as written and E ° cell = - 2.61 V.
C) This reaction is spontaneous as written and E ° cell = 2.61 V.
D) This reaction is non-spontaneous as written and E ° cell = 2.61 V.
E) This reaction is non-spontaneous as written and E ° cell = - 3.49 V.
2 Li+ (aq) + Fe (s)→Fe2+ (aq) + 2 Li (s) Which statement below is true regarding (1) the spontaneity of this reaction and (2) the value of E ° cell?
E ° red(Li1+/Li) = - 3.05V and E ° red(Fe2+/Fe) = - 0.44 V
A) This reaction is spontaneous as written and E ° cell = - 2.61 V.
B) This reaction is non-spontaneous as written and E ° cell = - 2.61 V.
C) This reaction is spontaneous as written and E ° cell = 2.61 V.
D) This reaction is non-spontaneous as written and E ° cell = 2.61 V.
E) This reaction is non-spontaneous as written and E ° cell = - 3.49 V.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
67
Exhibit 18-3 Use the standard reduction potentials below to answer the following question(s).
Ce4+(aq) + e - →Ce3+(aq) E ° = 1.61 V
Fe3+(aq) + e - →Fe2+(aq) E ° = 0.77 V
I2(s) + 2 e - →2 I - (aq) E ° = 0.54 V
2 H+(aq) + 2 e - →H2(g) E ° = 0.000 V
Cd2+(aq) + 2 e - →Cd(s) E ° = - 0.40 V
U4+(aq) + e - →U3+(aq) E ° = - 0.61 V
CdCO3(s) + 2 e - →Cd(s) + CO32 - (aq) E ° = - 0.74 V
Zn2+(aq) + 2 e - →Zn(s) E ° = - 0.76 V
U3+(aq) + 3 e - →U(s) E ° = - 1.80 V
Mg2+(aq) + 2 e - →Mg(s) E ° = - 2.37 V
Refer to Exhibit 18-3. The strongest reducing agent in this series is:
A) Mg2+
B) Mg (s)
C) Ce4+
D) Ce3+
E) none of these
Ce4+(aq) + e - →Ce3+(aq) E ° = 1.61 V
Fe3+(aq) + e - →Fe2+(aq) E ° = 0.77 V
I2(s) + 2 e - →2 I - (aq) E ° = 0.54 V
2 H+(aq) + 2 e - →H2(g) E ° = 0.000 V
Cd2+(aq) + 2 e - →Cd(s) E ° = - 0.40 V
U4+(aq) + e - →U3+(aq) E ° = - 0.61 V
CdCO3(s) + 2 e - →Cd(s) + CO32 - (aq) E ° = - 0.74 V
Zn2+(aq) + 2 e - →Zn(s) E ° = - 0.76 V
U3+(aq) + 3 e - →U(s) E ° = - 1.80 V
Mg2+(aq) + 2 e - →Mg(s) E ° = - 2.37 V
Refer to Exhibit 18-3. The strongest reducing agent in this series is:
A) Mg2+
B) Mg (s)
C) Ce4+
D) Ce3+
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
68
Exhibit 18-2 Consider a voltaic cell that is constructed from a strip of Ag dipped in an AgNO3 solution and a Fe (s) strip dipped in a solution of FeCl2 to answer the following question(s). An external circuit and a salt bridge connect these two electrodes.
Refer to Exhibit 18-2. Which cell reaction ensues spontaneously in this voltaic cell?
A) Fe2+ (aq) + 2 Ag (s)→ Fe (s) + 2 Ag+ (aq)
B) Fe2+ (aq) + 2 Ag+ (aq)→ Fe (s) + 2 Ag (s)
C) Fe (s) + 2 Ag (s)→ Fe2+ (aq) + 2 Ag+ (aq)
D) Fe (s) + 2 Ag+ (aq)→ Fe2+ (aq) + 2 Ag (s)
E) Fe2+ (aq) + Fe (s)→ 2 Ag1+ (aq) + 2 Ag (s)
Refer to Exhibit 18-2. Which cell reaction ensues spontaneously in this voltaic cell?
A) Fe2+ (aq) + 2 Ag (s)→ Fe (s) + 2 Ag+ (aq)
B) Fe2+ (aq) + 2 Ag+ (aq)→ Fe (s) + 2 Ag (s)
C) Fe (s) + 2 Ag (s)→ Fe2+ (aq) + 2 Ag+ (aq)
D) Fe (s) + 2 Ag+ (aq)→ Fe2+ (aq) + 2 Ag (s)
E) Fe2+ (aq) + Fe (s)→ 2 Ag1+ (aq) + 2 Ag (s)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
69
A standard cell with the reaction 2 H+ + 2 Cr2+→H2 (g) + 2 Cr3+ has a potential of 0.44 V. What is the standard reduction potential for the half-reaction:
Cr3+ + e - →Cr2+
A) +0.44 V
B) +0.22 V
C) - 0.22 V
D) - 0.44 V
E) not enough information
Cr3+ + e - →Cr2+
A) +0.44 V
B) +0.22 V
C) - 0.22 V
D) - 0.44 V
E) not enough information

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
70
Exhibit 18-3 Use the standard reduction potentials below to answer the following question(s).
Ce4+(aq) + e - →Ce3+(aq) E ° = 1.61 V
Fe3+(aq) + e - →Fe2+(aq) E ° = 0.77 V
I2(s) + 2 e - →2 I - (aq) E ° = 0.54 V
2 H+(aq) + 2 e - →H2(g) E ° = 0.000 V
Cd2+(aq) + 2 e - →Cd(s) E ° = - 0.40 V
U4+(aq) + e - →U3+(aq) E ° = - 0.61 V
CdCO3(s) + 2 e - →Cd(s) + CO32 - (aq) E ° = - 0.74 V
Zn2+(aq) + 2 e - →Zn(s) E ° = - 0.76 V
U3+(aq) + 3 e - →U(s) E ° = - 1.80 V
Mg2+(aq) + 2 e - →Mg(s) E ° = - 2.37 V
Refer to Exhibit 18-3. Which one of the following reactions proceeds spontaneously to the right when all reactants and products are in their standard states?
A) 2 Fe3+ (aq) + 2 I - (aq)→ 2 Fe2+ (aq) + I2 (s)
B) Cd (s) + Zn2+ (aq)→ Cd2+ (aq) + Zn (s)
C) 4 U3+ (aq)→ 3 U4+ (aq) + U (s)
D) 2 U4+ (aq) + Cd2+ (aq)→ 2 U3+ (aq) + Cd (s)
E) none of these
Ce4+(aq) + e - →Ce3+(aq) E ° = 1.61 V
Fe3+(aq) + e - →Fe2+(aq) E ° = 0.77 V
I2(s) + 2 e - →2 I - (aq) E ° = 0.54 V
2 H+(aq) + 2 e - →H2(g) E ° = 0.000 V
Cd2+(aq) + 2 e - →Cd(s) E ° = - 0.40 V
U4+(aq) + e - →U3+(aq) E ° = - 0.61 V
CdCO3(s) + 2 e - →Cd(s) + CO32 - (aq) E ° = - 0.74 V
Zn2+(aq) + 2 e - →Zn(s) E ° = - 0.76 V
U3+(aq) + 3 e - →U(s) E ° = - 1.80 V
Mg2+(aq) + 2 e - →Mg(s) E ° = - 2.37 V
Refer to Exhibit 18-3. Which one of the following reactions proceeds spontaneously to the right when all reactants and products are in their standard states?
A) 2 Fe3+ (aq) + 2 I - (aq)→ 2 Fe2+ (aq) + I2 (s)
B) Cd (s) + Zn2+ (aq)→ Cd2+ (aq) + Zn (s)
C) 4 U3+ (aq)→ 3 U4+ (aq) + U (s)
D) 2 U4+ (aq) + Cd2+ (aq)→ 2 U3+ (aq) + Cd (s)
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
71
A voltaic cell is based on a Co2+/Co half-cell and an Ag1+/Ag half-cell. Given E ° red(Co2+/Co) = - 0.277 V and E ° red(Ag1+/Ag) = +0.222 V, which statement below is true regarding which half cell operates at the positive electrode and what is the standard cell potential E ° cell for this voltaic cell?
A) The Co2+/Co half-cell operates at the positive electrode and the standard cell potential E ° cell is 0.499 V.
B) The Co2+/Co half-cell operates at the positive electrode and the standard cell potential E ° cell is - 0.055 V.
C) The Co2+/Co half-cell operates at the positive electrode and the standard cell potential E ° cell is 0.055 V.
D) The Ag1+/Ag half-cell operates at the positive electrode and the standard cell potential E ° cell is 0.499 V.
E) The Ag1+/Ag half-cell operates at the positive electrode and the standard cell potential E ° cell is 0.055 V.
A) The Co2+/Co half-cell operates at the positive electrode and the standard cell potential E ° cell is 0.499 V.
B) The Co2+/Co half-cell operates at the positive electrode and the standard cell potential E ° cell is - 0.055 V.
C) The Co2+/Co half-cell operates at the positive electrode and the standard cell potential E ° cell is 0.055 V.
D) The Ag1+/Ag half-cell operates at the positive electrode and the standard cell potential E ° cell is 0.499 V.
E) The Ag1+/Ag half-cell operates at the positive electrode and the standard cell potential E ° cell is 0.055 V.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
72
The E ° for a particular electrochemical reaction was found to be E ° = +1.086 V. This value of E ° means that under standard conditions this reaction is:
A) nonspontaneous
B) spontaneous
C) at equilibrium
D) exothermic
E) endothermic
A) nonspontaneous
B) spontaneous
C) at equilibrium
D) exothermic
E) endothermic

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
73
Given:
Ag+1 (aq) + e - ?Ag (s) E ° = 0.800 V Cl2 (g) + 2 e - ?2 Cl - (aq) E ° = +1.36 V Zn2+ (aq) + 2 e - ?Zn (s) E ° = - 0.76 V What is the proper arrangement for the order of increasing strength as a reducing agent ?
Ag (s), Cl - (aq) and Zn (s)
A) (weakest reducing agent) Ag (s) - (aq) < Zn (s) (strongest reducing agent)
B) (weakest reducing agent) Cl - (aq) < Ag (s) < Zn (s) (strongest reducing agent)
C) (weakest reducing agent) Zn (s) - (aq) < Ag (s) (strongest reducing agent)
D) (weakest reducing agent) Ag (s) - (aq) (strongest reducing agent)
E) (weakest reducing agent) Cl - (aq) < Zn (s) < Ag (s) (strongest reducing agent)
Ag+1 (aq) + e - ?Ag (s) E ° = 0.800 V Cl2 (g) + 2 e - ?2 Cl - (aq) E ° = +1.36 V Zn2+ (aq) + 2 e - ?Zn (s) E ° = - 0.76 V What is the proper arrangement for the order of increasing strength as a reducing agent ?
Ag (s), Cl - (aq) and Zn (s)
A) (weakest reducing agent) Ag (s) - (aq) < Zn (s) (strongest reducing agent)
B) (weakest reducing agent) Cl - (aq) < Ag (s) < Zn (s) (strongest reducing agent)
C) (weakest reducing agent) Zn (s) - (aq) < Ag (s) (strongest reducing agent)
D) (weakest reducing agent) Ag (s) - (aq) (strongest reducing agent)
E) (weakest reducing agent) Cl - (aq) < Zn (s) < Ag (s) (strongest reducing agent)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
74
The E ° for a particular electrochemical reaction was found to be E ° = +1.38 V. This value of E ° means that this reaction is
A) spontaneous and D G ° is negative.
B) nonspontaneous and D G ° is positive.
C) in an equilibrium state and D G ° is zero.
D) exothermic and D H ° is negative.
E) endothermic and D H ° is positive.
A) spontaneous and D G ° is negative.
B) nonspontaneous and D G ° is positive.
C) in an equilibrium state and D G ° is zero.
D) exothermic and D H ° is negative.
E) endothermic and D H ° is positive.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
75
Exhibit 18-3 Use the standard reduction potentials below to answer the following question(s).
Ce4+(aq) + e - →Ce3+(aq) E ° = 1.61 V
Fe3+(aq) + e - →Fe2+(aq) E ° = 0.77 V
I2(s) + 2 e - →2 I - (aq) E ° = 0.54 V
2 H+(aq) + 2 e - →H2(g) E ° = 0.000 V
Cd2+(aq) + 2 e - →Cd(s) E ° = - 0.40 V
U4+(aq) + e - →U3+(aq) E ° = - 0.61 V
CdCO3(s) + 2 e - →Cd(s) + CO32 - (aq) E ° = - 0.74 V
Zn2+(aq) + 2 e - →Zn(s) E ° = - 0.76 V
U3+(aq) + 3 e - →U(s) E ° = - 1.80 V
Mg2+(aq) + 2 e - →Mg(s) E ° = - 2.37 V
Refer to Exhibit 18-3. The strongest oxidizing agent in this list is:
A) Mg2+
B) Mg (s)
C) Ce4+
D) Ce3+
E) none of these
Ce4+(aq) + e - →Ce3+(aq) E ° = 1.61 V
Fe3+(aq) + e - →Fe2+(aq) E ° = 0.77 V
I2(s) + 2 e - →2 I - (aq) E ° = 0.54 V
2 H+(aq) + 2 e - →H2(g) E ° = 0.000 V
Cd2+(aq) + 2 e - →Cd(s) E ° = - 0.40 V
U4+(aq) + e - →U3+(aq) E ° = - 0.61 V
CdCO3(s) + 2 e - →Cd(s) + CO32 - (aq) E ° = - 0.74 V
Zn2+(aq) + 2 e - →Zn(s) E ° = - 0.76 V
U3+(aq) + 3 e - →U(s) E ° = - 1.80 V
Mg2+(aq) + 2 e - →Mg(s) E ° = - 2.37 V
Refer to Exhibit 18-3. The strongest oxidizing agent in this list is:
A) Mg2+
B) Mg (s)
C) Ce4+
D) Ce3+
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
76
Under standard conditions the voltaic cell with the reaction H2 (g) + 2 Fe3+→2 Fe2+ + 2 H+ has a potential of 0.77 V. What is the standard reduction potential for the half-reaction:
Fe3+ + e - →Fe2+
A) +0.77 V
B) +0.38 V
C) - 0.38 V
D) - 0.77 V
E) not enough information
Fe3+ + e - →Fe2+
A) +0.77 V
B) +0.38 V
C) - 0.38 V
D) - 0.77 V
E) not enough information

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
77
Given:
F2 (g) + 2 e - ?2 F - E ° = +2.87 V Cr2O 72 - (aq) + 14 H+ (aq) + 6 e - ?2 Cr3+ (aq) + 7 H2O E ° = 1.33 V Li+ (aq) + 1 e - (aq)?Li (s) E ° = - 3.05 V What is the proper arrangement for the order of increasing strength as an oxidizing agent ?
F2 (g), Cr2O72 - (aq) and Li+ (aq)
A) (weakest oxidizing agent) F2 (g) - (aq) < Li+ (aq) (strongest)
B) (weakest oxidizing agent) Cr2O72 - (aq) < F2 (g) < Li+ (aq) (strongest)
C) (weakest oxidizing agent) Cr2O72 - (aq) < Li+ (aq) < F2 (g) (strongest)
D) (weakest oxidizing agent) Li+ (aq) - (aq) (strongest)
E) (weakest oxidizing agent) Li+ (aq) - (aq) < F2 (g) (strongest)
F2 (g) + 2 e - ?2 F - E ° = +2.87 V Cr2O 72 - (aq) + 14 H+ (aq) + 6 e - ?2 Cr3+ (aq) + 7 H2O E ° = 1.33 V Li+ (aq) + 1 e - (aq)?Li (s) E ° = - 3.05 V What is the proper arrangement for the order of increasing strength as an oxidizing agent ?
F2 (g), Cr2O72 - (aq) and Li+ (aq)
A) (weakest oxidizing agent) F2 (g) - (aq) < Li+ (aq) (strongest)
B) (weakest oxidizing agent) Cr2O72 - (aq) < F2 (g) < Li+ (aq) (strongest)
C) (weakest oxidizing agent) Cr2O72 - (aq) < Li+ (aq) < F2 (g) (strongest)
D) (weakest oxidizing agent) Li+ (aq) - (aq) (strongest)
E) (weakest oxidizing agent) Li+ (aq) - (aq) < F2 (g) (strongest)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following is associated with a redox reaction that would proceed spontaneously towards the product side of the equation without having any outside work supplied to the system?
I. D G
II. E cell > 0
III. Q / K
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. D G
II. E cell > 0
III. Q / K
A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
79
Given:
2 Fe3+ + Cu (s)→Cu2+ + 2 Fe2+ E ° = +0.43 V 2 Ag+ + Cu (s)→Cu2+ + 2 Ag (s) E ° = +0.46 V What is the standard potential for the cell reaction in the following reaction?
Fe2+ + Ag+→Ag (s) + Fe3+
A) +0.89 V
B) +0.44 V
C) +0.03 V
D) - 0.03 V
E) none of these
2 Fe3+ + Cu (s)→Cu2+ + 2 Fe2+ E ° = +0.43 V 2 Ag+ + Cu (s)→Cu2+ + 2 Ag (s) E ° = +0.46 V What is the standard potential for the cell reaction in the following reaction?
Fe2+ + Ag+→Ag (s) + Fe3+
A) +0.89 V
B) +0.44 V
C) +0.03 V
D) - 0.03 V
E) none of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
80
The E ° for a particular electrochemical reaction was found to be E ° = - 0.86 V. This value of E ° means that under standard conditions this reaction is:
A) nonspontaneous
B) spontaneous
C) at equilibrium
D) exothermic
E) endothermic
A) nonspontaneous
B) spontaneous
C) at equilibrium
D) exothermic
E) endothermic

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck


