Deck 21: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/89
Play
Full screen (f)
Deck 21: Nuclear Chemistry
1
The transformation of 14C→14N is an example of:
A) a decay.
B)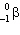 decay.
decay.
C)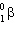 decay.
decay.
D) fusion
E) none of these
A) a decay.
B)
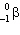 decay.
decay.C)
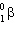 decay.
decay.D) fusion
E) none of these
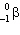 decay.
decay. 2
The alpha decay of 231Pa produces:
A) "227Ac."
B) "229Fr."
C) "227Np."
D) "229U."
E) "none of these"
A) "227Ac."
B) "229Fr."
C) "227Np."
D) "229U."
E) "none of these"
"227Ac."
3
Which form of radioactive decay can penetrate skin?
I. Gamma radiation
II. Beta particles
III. Alpha particles
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. Gamma radiation
II. Beta particles
III. Alpha particles
A) I only
B) II only
C) III only
D) I and II
E) All of these
I and II
4
The type of radioactivity that removes excess energy from the nucleus without changing the atomic number or mass number is called:
A) alpha decay.
B) beta decay.
C) gamma decay.
D) electron capture.
E) none of these
A) alpha decay.
B) beta decay.
C) gamma decay.
D) electron capture.
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
5
When a nuclide with atomic number = Z and mass number = A undergoes 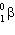 decay, the product nucleus will have:
decay, the product nucleus will have:
A) atomic number = Z, mass number = A
B) atomic number = Z - 2, mass number = A - 4.
C) atomic number = Z + 1, mass number = A.
D) atomic number = Z - 1, mass number = A.
E) none of these
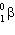 decay, the product nucleus will have:
decay, the product nucleus will have:A) atomic number = Z, mass number = A
B) atomic number = Z - 2, mass number = A - 4.
C) atomic number = Z + 1, mass number = A.
D) atomic number = Z - 1, mass number = A.
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
6
The most common mode of decay for elements with Z > 83 is:
A) alpha decay.
B) beta decay.
C) gamma decay.
D) electron capture.
E) none of these
A) alpha decay.
B) beta decay.
C) gamma decay.
D) electron capture.
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
7
The atomic mass of beryllium is 9.012. The radioactive nuclide 11Be is expected to decay by:
A) alpha emission.
B)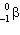 emission.
emission.
C) gamma emission.
D) electron capture.
E) none of these
A) alpha emission.
B)
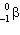 emission.
emission.C) gamma emission.
D) electron capture.
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
8
An isotope with Z = 34 is above the band of stability in a graph of the number of neutrons versus the number of protons. This nuclide decays by
A) alpha decay.
B)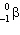 decay.
decay.
C) gamma decay.
D) electron capture.
E) none of these
A) alpha decay.
B)
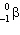 decay.
decay.C) gamma decay.
D) electron capture.
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
9
40K undergoes nuclear decay to 40Ar. Which decay process produces this product?
A) a decay
B)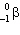 decay
decay
C)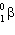 decay
decay
D) gamma decay
E) none of these
A) a decay
B)
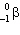 decay
decayC)
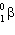 decay
decayD) gamma decay
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the three forms of radiation has the ability to penetrate a sheet of Aluminum that is 1.0 cm thick?
I. Alpha particles
II. Beta particles
III. Gamma rays
A) III only
B) I and II
C) I and III
D) All of these
E) None of these
I. Alpha particles
II. Beta particles
III. Gamma rays
A) III only
B) I and II
C) I and III
D) All of these
E) None of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
11
When 31 S undergoes 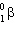 decay, the product nucleus is:
decay, the product nucleus is:
A) "31P"
B) "31Cl"
C) "31S"
D) "32S"
E) "none of these"
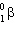 decay, the product nucleus is:
decay, the product nucleus is:A) "31P"
B) "31Cl"
C) "31S"
D) "32S"
E) "none of these"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
12
The product of a 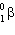 decay is 172 Ta. The nuclide that underwent decay is:
decay is 172 Ta. The nuclide that underwent decay is:
A) "172Ta."
B) "172Hf."
C) "172W."
D) "176Os."
E) "none of these"
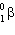 decay is 172 Ta. The nuclide that underwent decay is:
decay is 172 Ta. The nuclide that underwent decay is:A) "172Ta."
B) "172Hf."
C) "172W."
D) "176Os."
E) "none of these"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following components did Rutherford identify in radioactive decay?
A) "42 a particles"
B) "0 - 1 b particles"
C) "00 g rays"
D) "answer a and b"
E) "all of these"
A) "42 a particles"
B) "0 - 1 b particles"
C) "00 g rays"
D) "answer a and b"
E) "all of these"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
14
Which two natural radioactive decay processes will produce the same product nuclide?
A) a decay and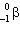 decay
decay
B)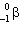 and electron capture
and electron capture
C)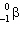 and
and 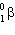
D)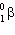 and electron capture
and electron capture
E) none of these
A) a decay and
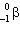 decay
decayB)
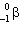 and electron capture
and electron captureC)
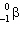 and
and 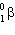
D)
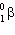 and electron capture
and electron captureE) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is positively charged?
A) "42 a particles"
B) "0 - 1 b particles"
C) "00 g rays"
D) "10n"
E) "None of these"
A) "42 a particles"
B) "0 - 1 b particles"
C) "00 g rays"
D) "10n"
E) "None of these"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
16
When 24 Na undergoes 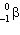 decay the nuclide produced is:
decay the nuclide produced is:
A) "24Na"
B) "24Mg"
C) "24Ne"
D) "23Ne"
E) "none of these"
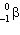 decay the nuclide produced is:
decay the nuclide produced is:A) "24Na"
B) "24Mg"
C) "24Ne"
D) "23Ne"
E) "none of these"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the three forms of radiation has no charge associated with it and is not deflected by the poles of a magnetic?
I. Alpha particles
II. Beta particles
III. Gamma rays
A) I only
B) II only
C) III only
D) I and II
E) II and III
I. Alpha particles
II. Beta particles
III. Gamma rays
A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
18
A radioactive element emits a 4He2+ from the nucleus. This is called:
A) alpha decay.
B) beta decay.
C) gamma decay.
D) electron capture.
E) none of these
A) alpha decay.
B) beta decay.
C) gamma decay.
D) electron capture.
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
19
The alpha decay of 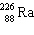 produces:
produces:
A) "222Pa."
B) "224Np."
C) "222Rn."
D) "224Po."
E) "none of these"
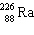 produces:
produces:A) "222Pa."
B) "224Np."
C) "222Rn."
D) "224Po."
E) "none of these"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
20
A radioactive element that emits an electron from the nucleus is said to undergo:
A) alpha decay.
B) beta decay.
C) gamma decay.
D) electron capture.
E) none of these
A) alpha decay.
B) beta decay.
C) gamma decay.
D) electron capture.
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
21
What daughter nuclide results after carbon - 14 undergoes beta decay ?
A) "147N"
B) "145B"
C) "156C"
D) "104Be"
E) "136C"
A) "147N"
B) "145B"
C) "156C"
D) "104Be"
E) "136C"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
22
Provided in the table below are two radionuclides and their corresponding half-life periods. Which radionuclide decays the fastest and which isotope is the least stable ?
Isotope Half-life period Lead - 210 22 Years Platinum - 190 6.9×1011 Years
A) Lead - 210 decays the fastest and is the least stable.
B) Lead - 210 decays the fastest and Platinum - 190 is the least stable.
C) Platinum - 190 decays the fastest and is the least stable.
D) Platinum - 190 decays the fastest and Lead - 210 is the least stable.
E) They both decay at the same rate and Lead - 210 is the least stable.
Isotope Half-life period Lead - 210 22 Years Platinum - 190 6.9×1011 Years
A) Lead - 210 decays the fastest and is the least stable.
B) Lead - 210 decays the fastest and Platinum - 190 is the least stable.
C) Platinum - 190 decays the fastest and is the least stable.
D) Platinum - 190 decays the fastest and Lead - 210 is the least stable.
E) They both decay at the same rate and Lead - 210 is the least stable.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
23
The decay rate of an alpha emitter sample is 975 disintegrations/minute. Exactly 1 hour later the sample decays at a rate of 644 disintegrations/minute. What is the half-life of this alpha emitter?
A) 100 min
B) 27.5 min
C) 2.79×10 - 2 min
D) 6.91×10 - 3 min
E) none of these
A) 100 min
B) 27.5 min
C) 2.79×10 - 2 min
D) 6.91×10 - 3 min
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
24
The nuclide 226 Ra undergoes a series of alpha and beta decays, finally producing stable 206 Pb. 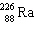
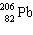 + A
+ A 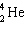 + B
+ B 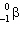 Give the values of A and B in the equation.
Give the values of A and B in the equation.
A) A = 5, B = 4
B) A = 4, B = 6
C) A = 6, B = 8
D) A = 8, B = 6
E) A = 4, B = 5
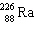
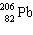 + A
+ A 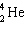 + B
+ B 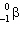 Give the values of A and B in the equation.
Give the values of A and B in the equation.A) A = 5, B = 4
B) A = 4, B = 6
C) A = 6, B = 8
D) A = 8, B = 6
E) A = 4, B = 5

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
25
Provided in the table below are two isotopes and their corresponding half-life periods. Which isotope decays the fastest and which isotope is the most stable ?
Isotope Half-life period Vanadium - 50 6.0×1015 Years Uranium - 235 7.1×108 Years
A) Vanadium - 50 decays the fastest and is the most stable.
B) Vanadium - 50 decays the fastest and Uranium - 235 is the most stable.
C) Uranium - 235 decays the fastest and is the most stable.
D) Uranium - 235 decays the fastest and Vanadium - 50 is the most stable.
E) They both decay at the same rate and Vanadium - 50 is the most stable.
Isotope Half-life period Vanadium - 50 6.0×1015 Years Uranium - 235 7.1×108 Years
A) Vanadium - 50 decays the fastest and is the most stable.
B) Vanadium - 50 decays the fastest and Uranium - 235 is the most stable.
C) Uranium - 235 decays the fastest and is the most stable.
D) Uranium - 235 decays the fastest and Vanadium - 50 is the most stable.
E) They both decay at the same rate and Vanadium - 50 is the most stable.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
26
Provided in the table below are two isotopes and their corresponding half-life periods. Which isotope decays the fastest and which isotope is the most stable ?
Isotope Half-life period Carbon - 14 5730 Years Lead - 210 22 Years
A) Carbon - 14 decays the fastest and is the most stable.
B) Carbon - 14 decays the fastest and Lead - 210 is the most stable.
C) Lead - 210 decays the fastest and is the most stable.
D) Lead - 210 decays the fastest and Carbon - 14 is the most stable.
E) They both decay at the same rate and Carbon - 14 is the most stable.
Isotope Half-life period Carbon - 14 5730 Years Lead - 210 22 Years
A) Carbon - 14 decays the fastest and is the most stable.
B) Carbon - 14 decays the fastest and Lead - 210 is the most stable.
C) Lead - 210 decays the fastest and is the most stable.
D) Lead - 210 decays the fastest and Carbon - 14 is the most stable.
E) They both decay at the same rate and Carbon - 14 is the most stable.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
27
A sample that contains 5.7×10 - 9 mol of 93Zr decays at a rate of 3.02×103 disintegrations per minute. What is the half-life of 93Zr?
(1 year = 5.256×105 minutes)
A) 1.0×106 years
B) 1.5×106 years
C) 2.2×106 years
D) The half-life cannot be found from this information.
E) none of these
(1 year = 5.256×105 minutes)
A) 1.0×106 years
B) 1.5×106 years
C) 2.2×106 years
D) The half-life cannot be found from this information.
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
28
Barium - 141 is a beta emitter. What is the daughter nuclide that is produced when barium - 141 undergoes beta emission?
A) "14157La"
B) "14256Ba"
C) "14155Cs"
D) "14558Ce"
E) "14552Te"
A) "14157La"
B) "14256Ba"
C) "14155Cs"
D) "14558Ce"
E) "14552Te"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
29
In the natural radioactive decay chain 238 U undergoes a series of radioactive decays, finally producing the stable nuclide 206 Pb:
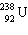
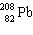 + A
+ A 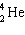 + B
+ B 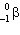 What are the values of A and B in this decay chain?
What are the values of A and B in this decay chain?
A) A = 10, B = 16
B) A = 8, B = 6
C) A = 6, B = 8
D) A = 16, B = 10
E) none of these
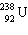
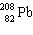 + A
+ A 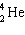 + B
+ B 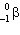 What are the values of A and B in this decay chain?
What are the values of A and B in this decay chain?A) A = 10, B = 16
B) A = 8, B = 6
C) A = 6, B = 8
D) A = 16, B = 10
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
30
Bismuth - 210, 210Bi, is an alpha emitter. What is the resulting daughter nuclide after Bismuth - 210 undergoes alpha emission?
A) "206Tl"
B) "214At"
C) "210Po"
D) "210Pb"
E) "206At"
A) "206Tl"
B) "214At"
C) "210Po"
D) "210Pb"
E) "206At"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
31
Consider comparing the two Phosphorous radionuclides given below and their corresponding half-life periods. Which statement below is true?
Isotope Half-life period Phosphorous - 28 0.28 seconds Phosphorous - 32 14.3 days
A) Phosphorous - 28 decays faster and is the most stable nuclide.
B) Phosphorous - 28 decays faster and is the least stable nuclide.
C) Phosphorous - 32 decays faster and is the most stable nuclide.
D) Phosphorous - 32 decays faster and is the least stable nuclide.
E) They both decay at the same rate but Phosphorous - 28 is more stable.
Isotope Half-life period Phosphorous - 28 0.28 seconds Phosphorous - 32 14.3 days
A) Phosphorous - 28 decays faster and is the most stable nuclide.
B) Phosphorous - 28 decays faster and is the least stable nuclide.
C) Phosphorous - 32 decays faster and is the most stable nuclide.
D) Phosphorous - 32 decays faster and is the least stable nuclide.
E) They both decay at the same rate but Phosphorous - 28 is more stable.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
32
When Plutonium - 239 changes to Uranium - 235, what form of radioactive decay is emitted?
A) alpha particles
B) beta particles
C) gamma rays
D) neutrons
E) positrons
A) alpha particles
B) beta particles
C) gamma rays
D) neutrons
E) positrons

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
33
A sample of a radioactive isotope decays at a rate of 988 disintegrations per minute. Exactly 85 minutes later the decay rate of the sample is 251 disintegrations per minute. What is the half-life of this isotope?
A) 1.61×10 - 2 min
B) 2.32×10 - 2 min
C) 15.0 min
D) 43.0 min
E) none of these
A) 1.61×10 - 2 min
B) 2.32×10 - 2 min
C) 15.0 min
D) 43.0 min
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
34
The nuclide that undergoes 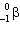 decay to form 72 Ge is:
decay to form 72 Ge is:
A) "72Ga."
B) "72As."
C) "76Se."
D) "73Ge."
E) "none of these"
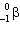 decay to form 72 Ge is:
decay to form 72 Ge is:A) "72Ga."
B) "72As."
C) "76Se."
D) "73Ge."
E) "none of these"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
35
The nuclide 226 Ra undergoes a series of alpha and beta decays, finally producing the stable nuclide 206 Pb. 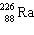
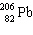 + A
+ A 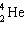 + B
+ B 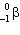 What are the values of A and B in the equation?
What are the values of A and B in the equation?
A) A = 6, B = 10
B) A = 5, B = 4
C) A = 4, B = 5
D) A = 10, B = 6
E) none of these
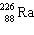
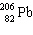 + A
+ A 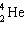 + B
+ B 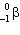 What are the values of A and B in the equation?
What are the values of A and B in the equation?A) A = 6, B = 10
B) A = 5, B = 4
C) A = 4, B = 5
D) A = 10, B = 6
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
36
The decay rate of a beta emitter sample is 1510 disintegrations/minute. Exactly 180 minutes later it decays at 963 disintegrations/minute. What is the half-life of this unstable nuclide?
A) 400 min
B) 277 min
C) 80 min
D) 2.50×10 - 3 min
E) none of these
A) 400 min
B) 277 min
C) 80 min
D) 2.50×10 - 3 min
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
37
The transformation 230Th→226Ra is an example of:
A) electron capture decay
B)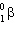 decay
decay
C)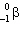 decay
decay
D) a decay.
E) none of these
A) electron capture decay
B)
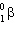 decay
decayC)
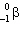 decay
decayD) a decay.
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
38
Bismuth - 214 is a radioactive beta emitter. What daughter nuclide is formed after Bismuth - 214 undergoes beta emission?
A) "21484Po"
B) "21482Pb"
C) "21885At"
D) "21081Tl"
E) "21085At"
A) "21484Po"
B) "21482Pb"
C) "21885At"
D) "21081Tl"
E) "21085At"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
39
Consider comparing the two radionuclides given below and their corresponding half-life periods. Which statement below is true?
Isotope Half-life period Carbon - 14 5730 years Iodine - 131 8 days
A) Carbon - 14 decays faster and is the most stable nuclide.
B) Carbon - 14 decays faster and is the least stable nuclide.
C) Iodine - 131 decays faster and is the most stable nuclide.
D) Iodine - 131 decays faster and is the least stable nuclide.
E) They both decay at the same rate but Carbon - 14 is more stable.
Isotope Half-life period Carbon - 14 5730 years Iodine - 131 8 days
A) Carbon - 14 decays faster and is the most stable nuclide.
B) Carbon - 14 decays faster and is the least stable nuclide.
C) Iodine - 131 decays faster and is the most stable nuclide.
D) Iodine - 131 decays faster and is the least stable nuclide.
E) They both decay at the same rate but Carbon - 14 is more stable.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
40
Polonium - 218 undergoes alpha emission. What is the daughter nuclide after polonium - 218 decays?

A) "21486Rn"
B) "22288Ra"
C) "21482Pb"
D) "21885At"
E) "21883Bi"

A) "21486Rn"
B) "22288Ra"
C) "21482Pb"
D) "21885At"
E) "21883Bi"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
41
A sample of parchment produces 10.3 disintegrations of 14C per minute per gram of carbon. Estimate the age of the parchment, assuming the original activity was 15.3 disintegrations per minute per gram of carbon. (t1/2 = 5730 years)
A) 8.5×103 yr
B) 3.9×103 yr
C) 3.3×103 yr
D) 2.3×103 yr
E) none of these
A) 8.5×103 yr
B) 3.9×103 yr
C) 3.3×103 yr
D) 2.3×103 yr
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
42
The half-life of Nitrogen - 16 is 7 seconds. How long does it take for 100 mg of Nitrogen - 16 to be reduced tO6.25 mg?
A) 1.75 seconds
B) 4 seconds
C) 5 seconds
D) 28 seconds
E) 35 seconds
A) 1.75 seconds
B) 4 seconds
C) 5 seconds
D) 28 seconds
E) 35 seconds

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
43
Iodine - 131 is a radionuclide that is frequently used in nuclear medicine. Among other things, it is used to detect fluid buildup in the brain. The half-life of iodine - 131 is 8.0 days. After introducing this radionuclide to a patient, if only 1/16th of the original sample remains, how many days have expired?
A) 0.50 days
B) 2 days
C) 7.5 days
D) 24 days
E) 32 days
A) 0.50 days
B) 2 days
C) 7.5 days
D) 24 days
E) 32 days

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
44
Phosphorous - 32 has a half-life of 14.3 days. How many days are required for 4.00 grams of phosphorous - 32 to decay to 1.00 gram?
A) 1.80 days
B) 16.1 days
C) 28.6 days
D) 42.9 days
E) 57.2 days
A) 1.80 days
B) 16.1 days
C) 28.6 days
D) 42.9 days
E) 57.2 days

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
45
Technetium - 99 has a half-life equal tO6.0 hours. What fraction of Technetiun - 99 remains undecayed after 18 hours?
A) 0.000
B) 1/3
C) 1/8
D) 1/16
E) 1/108
A) 0.000
B) 1/3
C) 1/8
D) 1/16
E) 1/108

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
46
What type of nuclear reaction is observed in the following process?
24896Cm + 2810Ne→11651Sb + 16055Cs
A) Radioactive decay
B) Alpha emission
C) Beta emission
D) Nuclear fission
E) Nuclear fusion
24896Cm + 2810Ne→11651Sb + 16055Cs
A) Radioactive decay
B) Alpha emission
C) Beta emission
D) Nuclear fission
E) Nuclear fusion

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
47
What type of nuclear reaction is illustrated below?
23592U + 10n→13954Xe + 9538Sr + 2 10n
A) Alpha emission
B) Beta emission
C) Radioactive decay
D) Nuclear fission
E) Nuclear fusion
23592U + 10n→13954Xe + 9538Sr + 2 10n
A) Alpha emission
B) Beta emission
C) Radioactive decay
D) Nuclear fission
E) Nuclear fusion

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
48
The half-life of 14C is 5730 years. Carbon - 14 dating would not be useful in the dating of which of the following samples?
A) "bones that are about 1000 years old"
B) "relics from 500 BC"
C) "paper from the 1970's"
D) "the remains of one of Columbus's crew (1492 AD)"
E) "14C dating could be used for all of these samples"
A) "bones that are about 1000 years old"
B) "relics from 500 BC"
C) "paper from the 1970's"
D) "the remains of one of Columbus's crew (1492 AD)"
E) "14C dating could be used for all of these samples"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
49
The reaction below can be best described as what type of nuclear reaction?
23592U + 10n→13956Ba + 9436Kr + 3 10n
A) Alpha decay
B) Beta decay
C) Nuclear fission
D) Nuclear fusion
E) Radioactive decay
23592U + 10n→13956Ba + 9436Kr + 3 10n
A) Alpha decay
B) Beta decay
C) Nuclear fission
D) Nuclear fusion
E) Radioactive decay

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following types of nuclear reactions is(are) considered bombardment reactions ?
I. Radioactive decay
II. Nuclear fission
III. Nuclear fusion
A) I only
B) II only
C) III only
D) II and III
E) All of these
I. Radioactive decay
II. Nuclear fission
III. Nuclear fusion
A) I only
B) II only
C) III only
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
51
Barium - 122 has a half-life of 2 minutes. You just obtained a sample weighing 10.0 grams. It takes 10 minutes to set up the experiment in which barium - 122 will be used. How many grams of barium - 122 will be left when you begin the experiment?
A) 0.00 g remain
B) 0.313 g remain
C) 0.625 g remain
D) 2.00 g remain
E) 50.0 g remain
A) 0.00 g remain
B) 0.313 g remain
C) 0.625 g remain
D) 2.00 g remain
E) 50.0 g remain

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
52
In a nuclear reaction, the nuclide 75As absorbs a neutron and emits a gamma ray. The product nucleus is:
A) "76Ge."
B) "76Se."
C) "72Ga."
D) "76As."
E) "none of these"
A) "76Ge."
B) "76Se."
C) "72Ga."
D) "76As."
E) "none of these"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
53
A Uranium ore contains 0.250 g of 206Pb per gram of Uranium. Assuming all of the lead was produced by decay of 238U (t1/2 = 4.5×109 years), what is the age of the rock?
A) 4.4×10 - 11 years
B) 8.1×108 years
C) 1.9×109 years
D) 3.2×109 years
E) 9.0×109 years
A) 4.4×10 - 11 years
B) 8.1×108 years
C) 1.9×109 years
D) 3.2×109 years
E) 9.0×109 years

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
54
A 4.00 gram sample of a radionuclide at room temperature has a half-life of 91.3 days. How much of the sample remains after 365 days?
A) 0.250 g
B) 2.00 g
C) 0.400 g
D) 4.00 g
E) 1.00 g
A) 0.250 g
B) 2.00 g
C) 0.400 g
D) 4.00 g
E) 1.00 g

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
55
The half-life of strontium - 90 is 28 years. How many grams of this nuclide in a 4.00 gram sample will remain after 112 years?
A) 0.00 g
B) 7.70×10 - 34 g
C) 1.49×10 - 8 g
D) 0.250 g
E) 1.00 g
A) 0.00 g
B) 7.70×10 - 34 g
C) 1.49×10 - 8 g
D) 0.250 g
E) 1.00 g

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
56
Assuming that the original activity of carbon - 14 was 15.3 disintegrations/min/g, what is the present disintegration rate (in disintegrations/min/g) in a sample of parchment that is 2.10×103 years old?
(t1/2 = 5730 years)
A) 5.6
B) 11.9
C) 15.3
D) 41.7
E) none of these
(t1/2 = 5730 years)
A) 5.6
B) 11.9
C) 15.3
D) 41.7
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
57
What type of nuclear reaction is illustrated in the following transformation?
23592U + 10n→14455Cs + 9037Rb + 2 10n
A) Alpha decay
B) Beta decay
C) Nuclear fission
D) Nuclear fusion
E) Radioactive decay
23592U + 10n→14455Cs + 9037Rb + 2 10n
A) Alpha decay
B) Beta decay
C) Nuclear fission
D) Nuclear fusion
E) Radioactive decay

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
58
What type of nuclear reaction is illustrated below?
31H + 21H→42He + 10n
A) Alpha emission
B) Beta emission
C) Radioactive decay
D) Nuclear fission
E) Nuclear fusion
31H + 21H→42He + 10n
A) Alpha emission
B) Beta emission
C) Radioactive decay
D) Nuclear fission
E) Nuclear fusion

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
59
Cobalt - 60 has a half-life equal tO5 .3 years. What fraction of cobalt - 60 remains undecayed after 21.2 years?
A) 0.000
B) 0.0625
C) 0.125
D) 0.250
E) 0.500
A) 0.000
B) 0.0625
C) 0.125
D) 0.250
E) 0.500

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
60
Polonium - 218, a decay product of radon - 222, has a half-life of 3 minutes. What percentage of the polonium - 218 formed will remain in the lung 9 minutes after inhalation?
A) 0%
B) 12.5%
C) 25%
D) 75%
E) 87.5%
A) 0%
B) 12.5%
C) 25%
D) 75%
E) 87.5%

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
61
What is the missing particle in the nuclear reaction?
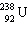 +
+ 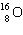
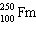 + ____
+ ____
A)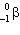
B)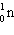
C)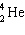
D)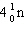
E) none of these
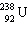 +
+ 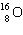
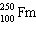 + ____
+ ____A)
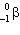
B)
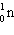
C)
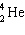
D)
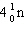
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
62
How many neutrons are produced in the following nuclear reaction involving Uranium - 235?
23592U + 10n→7230Zn + 16062Sm + ??
A) 0 10n
B) 1 10n
C) 2 10n
D) 3 10n
E) 4 10n
23592U + 10n→7230Zn + 16062Sm + ??
A) 0 10n
B) 1 10n
C) 2 10n
D) 3 10n
E) 4 10n

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
63
The mass of an 1H atom is 1.007825 u, and a neutron has a mass of 1.008665 u. The atomic mass of 23Na is 22.9898 u. What is the mass defect of this nuclide in u?
A) 0.2003 u
B) 0.1994 u
C) 0.1901 u
D) 0.1892 u
E) none of these
A) 0.2003 u
B) 0.1994 u
C) 0.1901 u
D) 0.1892 u
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
64
In any nuclear decay:
A) The mass of the products is always greater than that of the reactant.
B) The mass of the products is always less than that of the reactant.
C) The mass of the products is equal to that of the reactant.
D) There is no relationship between the masses of product and reactant.
E) none of these
A) The mass of the products is always greater than that of the reactant.
B) The mass of the products is always less than that of the reactant.
C) The mass of the products is equal to that of the reactant.
D) There is no relationship between the masses of product and reactant.
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
65
What must be placed in the blank to produce a balanced nuclear equation?
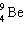 +
+ 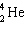 ____ +
____ + 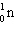
A)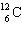
B)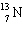
C)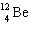
D)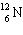
E) none of these
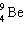 +
+ 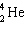 ____ +
____ + 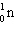
A)
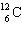
B)
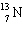
C)
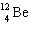
D)
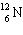
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
66
What is the missing particle in the nuclear equation?
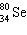 + ____
+ ____ 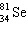 +
+ 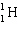
A)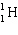
B)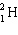
C)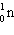
D)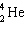
E) none of these
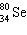 + ____
+ ____ 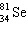 +
+ 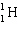
A)
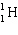
B)
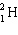
C)
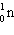
D)
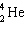
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
67
The mass defect of 31P is 0.2822 u. The binding energy per nucleon (in MeV) for this atom is:
A) 8.480
B) 17.52
C) 16.43
D) 262.9
E) none of these
A) 8.480
B) 17.52
C) 16.43
D) 262.9
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
68
The mass defect of 23Na is 0.2003 u. The binding energy per nucleon (in MeV) for this atom is:
A) 186
B) 8.112
C) 16.96
D) 15.54
E) none of these
A) 186
B) 8.112
C) 16.96
D) 15.54
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
69
The mass defect of 19F is 0.1587 u. The binding energy per nucleon (in MeV) for this atom is:
A) 147.8
B) 16.42
C) 7.780
D) 14.78
E) none of these
A) 147.8
B) 16.42
C) 7.780
D) 14.78
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
70
What must be placed in the blank to produce a balanced nuclear equation?
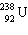 +
+ 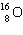 ____ +
____ + 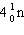
A)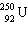
B)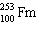
C)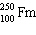
D)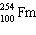
E) none of these
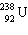 +
+ 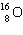 ____ +
____ + 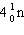
A)
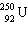
B)
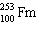
C)
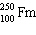
D)
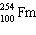
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
71
Curium - 248, 248Cm, was bombarded, yielding Antimony - 116, 116Sb, and Cesium - 160, 160Cs. What was the bombarding nucleus?
A) "7 42 a"
B) "28Ne"
C) "28Ni"
D) "28Si"
E) "10B"
A) "7 42 a"
B) "28Ne"
C) "28Ni"
D) "28Si"
E) "10B"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
72
One of the fission reactions that occurs in uranium reactors is:
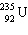 +
+ 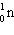
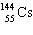 +
+ 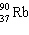 + ____ To balance this nuclear equation the blank is:
+ ____ To balance this nuclear equation the blank is:
A) "10n"
B) "2 10n"
C) "42He"
D) "0 - 1 b"
E) "none of these"
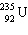 +
+ 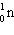
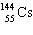 +
+ 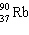 + ____ To balance this nuclear equation the blank is:
+ ____ To balance this nuclear equation the blank is:A) "10n"
B) "2 10n"
C) "42He"
D) "0 - 1 b"
E) "none of these"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
73
What must be placed in the blank to produce a balanced nuclear equation?
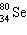 +
+ 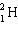 ____ +
____ + 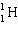
A)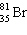
B)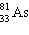
C)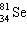
D)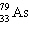
E) none of these
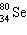 +
+ 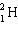 ____ +
____ + 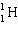
A)
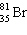
B)
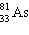
C)
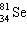
D)
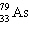
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
74
How many alpha particles are produced in the following nuclear bombardment reaction?
23090Th + 11p→22387Fr + ?
42 a
A) zero
B) one
C) two
D) three
E) four
23090Th + 11p→22387Fr + ?
42 a
A) zero
B) one
C) two
D) three
E) four

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
75
The binding energy per nucleon is greatest for:
A) the very light atoms.
B) the very heavy atoms.
C) atoms with odd numbers of both neutrons and protons.
D) the stable isotopes of elements near iron.
E) none of these
A) the very light atoms.
B) the very heavy atoms.
C) atoms with odd numbers of both neutrons and protons.
D) the stable isotopes of elements near iron.
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
76
What is the missing symbol in the following nuclear equation?
2713Al + 21H→???
+ 42 a
A) "2513Al"
B) "2512Mg"
C) "3312Mg"
D) "2516S"
E) "3316S"
2713Al + 21H→???
+ 42 a
A) "2513Al"
B) "2512Mg"
C) "3312Mg"
D) "2516S"
E) "3316S"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
77
The mass of an 1H atom is 1.007825 u, and a neutron has a mass of 1.008665 u. The atomic mass of 31P is 30.9738 u. What is the mass defect of this nuclide in u?
A) 0.2560 u
B) 0.2813 u
C) 0.2822 u
D) 0.2686 u
E) none of these
A) 0.2560 u
B) 0.2813 u
C) 0.2822 u
D) 0.2686 u
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
78
What is the missing symbol in the following nuclear reaction?
20983Bi + 42He→???
+ 3 10n
A) "21085At"
B) "21083Bi"
C) "21082Pb"
D) "21285At"
E) "21283Bi"
20983Bi + 42He→???
+ 3 10n
A) "21085At"
B) "21083Bi"
C) "21082Pb"
D) "21285At"
E) "21283Bi"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
79
The mass of an 1H atom is 1.007825 u, and a neutron has a mass of 1.008665 u. What is the mass defect (in u) of 199F (atomic mass = 18.9984 u)?
A) 0.1502 u
B) 0.1577 u
C) 0.1587 u
D) 0.1662 u
E) none of these
A) 0.1502 u
B) 0.1577 u
C) 0.1587 u
D) 0.1662 u
E) none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
80
One of the fission reactions that occurs in uranium reactors is:
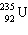 +
+ 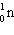
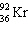 +
+  + ____ To balance this nuclear equation the blank is:
+ ____ To balance this nuclear equation the blank is:
A) "10n"
B) "3 10n"
C) "42He"
D) "31H"
E) "none of these"
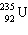 +
+ 
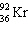 +
+  + ____ To balance this nuclear equation the blank is:
+ ____ To balance this nuclear equation the blank is:A) "10n"
B) "3 10n"
C) "42He"
D) "31H"
E) "none of these"

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck


