Deck 22: Organic Chemistry and Biochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/175
Play
Full screen (f)
Deck 22: Organic Chemistry and Biochemistry
1
Which compound below is named nonane ?
A) CH4
B) CH3CH2CH3
C) CH3(CH2)3CH3
D) CH3(CH2)5CH3
E) CH3(CH2)7CH3
A) CH4
B) CH3CH2CH3
C) CH3(CH2)3CH3
D) CH3(CH2)5CH3
E) CH3(CH2)7CH3
CH3(CH2)7CH3
2
Which molecule listed below (none are cyclic) is a saturated hydrocarbon?
A) C2H4
B) C3H6
C) C4H8
D) C5H8
E) none of these
A) C2H4
B) C3H6
C) C4H8
D) C5H8
E) none of these
none of these
3
The molecular formula for hexane is:
A) C6H6
B) C6H8
C) C6H12
D) C6H14
E) none of these
A) C6H6
B) C6H8
C) C6H12
D) C6H14
E) none of these
C6H14
4
What is the correct IUPAC name for the following branching alkane ?
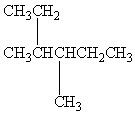
A) 2-Ethyl-3-methylpentane
B) 2-sec-butylbutane
C) 3,4-Dimethylhexane
D) 4-Ethyl-3-methylpentane
E) 2,3-Diethylbutane

A) 2-Ethyl-3-methylpentane
B) 2-sec-butylbutane
C) 3,4-Dimethylhexane
D) 4-Ethyl-3-methylpentane
E) 2,3-Diethylbutane

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
5
What attribute of carbon is false ?
A) It can form extended chains or rings with itself.
B) It can be involved in a single, double or triple bond.
C) It can bond up to five other atoms.
D) As a central atom, its electron pair geometry can be tetrahedral, trigonal planar or linear.
E) Carbon forms compounds in which its X - C - X bond angles are either 109.5 ° , 120 ° or 180 ° .
A) It can form extended chains or rings with itself.
B) It can be involved in a single, double or triple bond.
C) It can bond up to five other atoms.
D) As a central atom, its electron pair geometry can be tetrahedral, trigonal planar or linear.
E) Carbon forms compounds in which its X - C - X bond angles are either 109.5 ° , 120 ° or 180 ° .

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
6
The name of the compound pictured is:
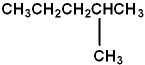
A) 1-methylpentane
B) 2-methylpentane
C) 4-methylpentane
D) 2-hexane
E) none of these
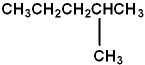
A) 1-methylpentane
B) 2-methylpentane
C) 4-methylpentane
D) 2-hexane
E) none of these

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
7
What is the correct systematic IUPAC name for the following branching alkane ?
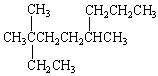
A) 2-Ethyl-2,5-Dimethyloctane
B) 3,3,6-Trimethylnonane
C) Dodecane
D) 2-Ethyl-2-methyl-5-propylhexane
E) 2-Propyl-5-ethyl-5-methylhexane

A) 2-Ethyl-2,5-Dimethyloctane
B) 3,3,6-Trimethylnonane
C) Dodecane
D) 2-Ethyl-2-methyl-5-propylhexane
E) 2-Propyl-5-ethyl-5-methylhexane

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
8
What is the IUPAC name for the following alkane?
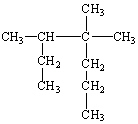
A) 2-Ethyl-3-Methyl-3-Propylbutane
B) 3-Ethyl-2-Methyl-2-Propylbutane
C) 2-Ethyl-3,3-Dimethylhexane
D) 2- sec -butyl-2-methylpentane
E) 3,4,4-Trimethylheptane

A) 2-Ethyl-3-Methyl-3-Propylbutane
B) 3-Ethyl-2-Methyl-2-Propylbutane
C) 2-Ethyl-3,3-Dimethylhexane
D) 2- sec -butyl-2-methylpentane
E) 3,4,4-Trimethylheptane

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
9
What is the general formula for any continuous chain alkane?
A) CnH2n+2
B) CnH2n
C) CnH2n - 2
D) CnHn
E) CnHn+2
A) CnH2n+2
B) CnH2n
C) CnH2n - 2
D) CnHn
E) CnHn+2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
10
What is the IUPAC name for the following organic compound?
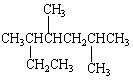
A) 2,4,5-Trimethylheptane
B) 3,4,6-Trimethylheptane
C) Decane
D) 2-Ethyl-3,5-dimethylhexane
E) 5-Ethyl-2,4-dimethylhexane

A) 2,4,5-Trimethylheptane
B) 3,4,6-Trimethylheptane
C) Decane
D) 2-Ethyl-3,5-dimethylhexane
E) 5-Ethyl-2,4-dimethylhexane

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
11
What is the correct IUPAC name for the following compound?
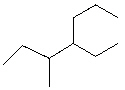
A) 3-methyl-4-propylhexane
B) 3-propyl-4-methylhexane
C) 3-methyl-4-ethylheptane
D) 4-ethyl-3-methylheptane
E) 2-Ethyl-3-propylpentane

A) 3-methyl-4-propylhexane
B) 3-propyl-4-methylhexane
C) 3-methyl-4-ethylheptane
D) 4-ethyl-3-methylheptane
E) 2-Ethyl-3-propylpentane

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
12
Consider the following molecules. Which of these molecules are considered saturated ?
A)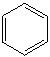
B) CH3CH3
C) CH3CH=CH2
D)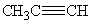
E) All of these are saturated
A)

B) CH3CH3
C) CH3CH=CH2
D)
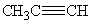
E) All of these are saturated

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
13
What can be stated about the name, 1,2,2-Trimethylpentane?
(Hint:
Draw the molecule from the name given and then try to rename it.)
A) 1,2,2-Trimethylpentane is a valid name for a compound.
B) This name is wrong because the longest chain is three carbon atoms and it should be named 2-Ethyl-2-Propylpropane.
C) This name is wrong because the longest chain is four carbons and it should be named 2-Methyl-2-propylbutane.
D) This name is wrong because the longest chain is five carbon atoms and it should be named 2-Ethyl-2-methylpentane
E) This name is wrong because the longest chain is six carbon atoms in length and it should be named 3,3-Dimethylhexane.
(Hint:
Draw the molecule from the name given and then try to rename it.)
A) 1,2,2-Trimethylpentane is a valid name for a compound.
B) This name is wrong because the longest chain is three carbon atoms and it should be named 2-Ethyl-2-Propylpropane.
C) This name is wrong because the longest chain is four carbons and it should be named 2-Methyl-2-propylbutane.
D) This name is wrong because the longest chain is five carbon atoms and it should be named 2-Ethyl-2-methylpentane
E) This name is wrong because the longest chain is six carbon atoms in length and it should be named 3,3-Dimethylhexane.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
14
Which line-angle drawing below is named Heptane ?
A)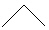
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
15
What is the IUPAC name for the straight chain alkane with a formula of CH3CH2CH2CH2CH3?
A) methane
B) propane
C) pentane
D) heptane
E) nonane
A) methane
B) propane
C) pentane
D) heptane
E) nonane

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
16
What can be stated about the name of 3-Methyl-4-Ethylhexane?
(Hint:
Draw the molecule from the name given and then try to rename it.)
A) This name is a valid name for a compound.
B) This name is incorrect because it provides the wrong numbering scheme. The compound should be named 3-Ethyl-4-Methylhexane.
C) This name is incorrect because the longest chain is five carbon atoms long and there is only one substituent. The name of the compound should be 3-sec-butylpentane.
D) This name is incorrect because the longest chain is five carbon atoms and there are two substituent groups. The name of the compound should be 2,3-Diethylpentane.
E) This name is incorrect. There are nine carbon atoms and therefore the compound should be named nonane.
(Hint:
Draw the molecule from the name given and then try to rename it.)
A) This name is a valid name for a compound.
B) This name is incorrect because it provides the wrong numbering scheme. The compound should be named 3-Ethyl-4-Methylhexane.
C) This name is incorrect because the longest chain is five carbon atoms long and there is only one substituent. The name of the compound should be 3-sec-butylpentane.
D) This name is incorrect because the longest chain is five carbon atoms and there are two substituent groups. The name of the compound should be 2,3-Diethylpentane.
E) This name is incorrect. There are nine carbon atoms and therefore the compound should be named nonane.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following compounds is considered unsaturated ?
I. CH ₃ CH₂ CH ₂ CH ₃
II. CH₂ =CHCH₃
III.
A) I only
B) II only
C) III only
D) I and II
E) II and III
I. CH ₃ CH₂ CH ₂ CH ₃
II. CH₂ =CHCH₃
III.

A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
18
Which organic compound listed below is octane ?
A) CH3(CH2)4CH3
B) CH3CH(CH3)CH2CH3
C) CH3(CH2)6CH3
D) (CH3)3CCH2C(CH3)3
E) CH3(CH2)8CH3
A) CH3(CH2)4CH3
B) CH3CH(CH3)CH2CH3
C) CH3(CH2)6CH3
D) (CH3)3CCH2C(CH3)3
E) CH3(CH2)8CH3

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following line angle drawings is pentane ?
A)
B)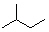
C)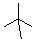
D)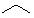
E)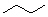
A)

B)

C)

D)

E)
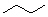

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
20
What is the IUPAC name for the following organic compound?
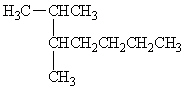
A) 1-methyl-1-propylpentane
B) 2-methyl-3-methylheptane
C) 2,3-dimethylheptane
D) 5,6-dimethylheptane
E) 2-butyl-3-methylbutane
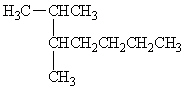
A) 1-methyl-1-propylpentane
B) 2-methyl-3-methylheptane
C) 2,3-dimethylheptane
D) 5,6-dimethylheptane
E) 2-butyl-3-methylbutane

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
21
Compare compounds I-III with compound A. Which compound(s) listed below is(are) the same compound as compound A?
(Hint:
Try to name each.)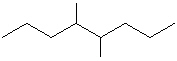
A
I.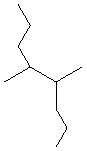
II.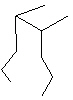
III.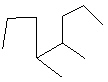
A) Compound I only
B) Compound II only
C) Compound III only
D) Compounds I and II
E) All of these
(Hint:
Try to name each.)

A
I.

II.

III.

A) Compound I only
B) Compound II only
C) Compound III only
D) Compounds I and II
E) All of these

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
22
Exhibit 22-1 Consider Compound A and Compounds I-III to answer the following question(s).
A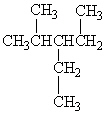
I.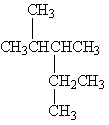
II.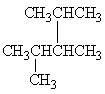
III.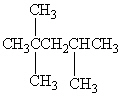
Refer to Exhibit 22-1. Which of the three compounds I-III is the same compound as compound A?
A) Compound I
B) Compound II
C) Compound III
D) Compounds I and II
E) All of these are the same compound as compound A
A

I.

II.

III.

Refer to Exhibit 22-1. Which of the three compounds I-III is the same compound as compound A?
A) Compound I
B) Compound II
C) Compound III
D) Compounds I and II
E) All of these are the same compound as compound A

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
23
How are the three alkanes shown below with a formula of C6 H 14 related?
(Hint:
Give them a name.)
I.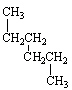
II.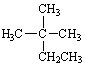
III.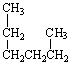
A) Structures I and II are different conformations of the same compound and structure III is a structural isomer of I and II.
B) Structures I and III are different conformations of the same compound and structure II is a structural isomer of I and III.
C) Structures II and III are different conformations of the same compound and structure I is a structural isomer of II and III.
D) All three structures are different conformations of the same compound.
E) All three structures are three unique structural isomers with the formula C6H14.
(Hint:
Give them a name.)
I.

II.

III.

A) Structures I and II are different conformations of the same compound and structure III is a structural isomer of I and II.
B) Structures I and III are different conformations of the same compound and structure II is a structural isomer of I and III.
C) Structures II and III are different conformations of the same compound and structure I is a structural isomer of II and III.
D) All three structures are different conformations of the same compound.
E) All three structures are three unique structural isomers with the formula C6H14.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
24
Exhibit 22-1 Consider Compound A and Compounds I-III to answer the following question(s).
A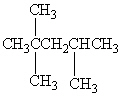
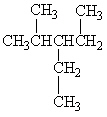
I.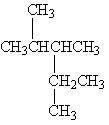
II.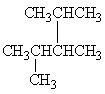
III.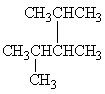
Refer to Exhibit 22-1. Which of the three compounds I-III are structural isomers of compound A?
A) Compound I
B) Compound II
C) Compound III
D) Compounds I and II
E) Compounds II and III
A


I.

II.

III.

Refer to Exhibit 22-1. Which of the three compounds I-III are structural isomers of compound A?
A) Compound I
B) Compound II
C) Compound III
D) Compounds I and II
E) Compounds II and III

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
25
Exhibit 22-1 Consider Compound A and Compounds I-III to answer the following question(s).
A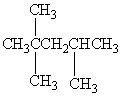
I.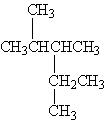
II.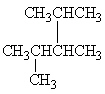
III.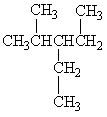
Refer to Exhibit 22-1. What is the systematic IUPAC name for compound A?
A) Octane
B) 2-Methyl-3-isopropylbutane
C) 2,3,4-Trimethylpentane
D) Propylpentane
E) 2-Ethyl-2,3-dimethylbutane
A

I.

II.

III.

Refer to Exhibit 22-1. What is the systematic IUPAC name for compound A?
A) Octane
B) 2-Methyl-3-isopropylbutane
C) 2,3,4-Trimethylpentane
D) Propylpentane
E) 2-Ethyl-2,3-dimethylbutane

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
26
What common alkyl group is shown below?
(The squiggly line represents a long straight chain.)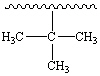
A) isopropyl
B) n-butyl
C) isobutyl
D) sec -butyl
E) tert -butyl
(The squiggly line represents a long straight chain.)

A) isopropyl
B) n-butyl
C) isobutyl
D) sec -butyl
E) tert -butyl

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
27
Which cyclic compound listed below is Cycloheptane ?
A)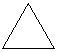
B)
C)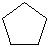
D)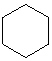
E)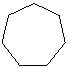
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
28
Compare compounds I-III with compound A. Which compound(s) is(are) the same as compound A?
(Hint:
Try to name each.)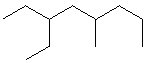
I.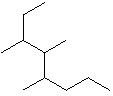
II.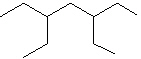
III.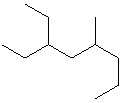
A) I only
B) II only
C) III only
D) I and II
E) None are the same as compound A
(Hint:
Try to name each.)

I.

II.

III.

A) I only
B) II only
C) III only
D) I and II
E) None are the same as compound A

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
29
Compare compounds I-III with compound A. Which of the three compounds listed below is(are) the same compound as compound A?
(Hint:
Try to give them each a name.)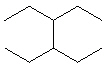
I.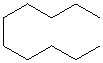
II.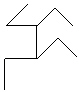
III.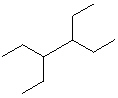
A) I only
B) II only
C) III only
D) I and II
E) II and III
(Hint:
Try to give them each a name.)

I.

II.

III.

A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
30
Compare compounds I-III with compound A. Which compound(s) listed below is(are) the same compound as compound A?
(Hint:
Try to name each.)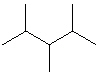
I.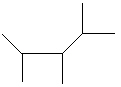
II.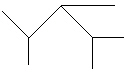
III.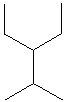
A) I only
B) II only
C) III only
D) I and II
E) All of these
(Hint:
Try to name each.)

I.

II.

III.

A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
31
What is the correct IUPAC name for the following cyclic alkane?
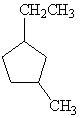
A) Cycloctane
B) Propylcyclopentane
C) 1-Ethyl-3-Methylcyclopentane
D) 1-Methyl-3-Ethylcyclopentane
E) Meta-Ethylmethylcyclopentane

A) Cycloctane
B) Propylcyclopentane
C) 1-Ethyl-3-Methylcyclopentane
D) 1-Methyl-3-Ethylcyclopentane
E) Meta-Ethylmethylcyclopentane

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
32
What common branching alkyl group is shown below?
(The horizontal squiggly line represents a long continuous straight chain.)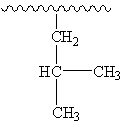
A) isopropyl
B) n-butyl
C) isobutyl
D) sec -butyl
E) tert -butyl
(The horizontal squiggly line represents a long continuous straight chain.)

A) isopropyl
B) n-butyl
C) isobutyl
D) sec -butyl
E) tert -butyl

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
33
Compare compounds I-III with compound A. Which of the three compounds listed below is(are) the same compound as compound A?
(Hint:
Try to give them each a name.)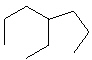
I.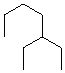
II.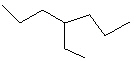
III.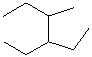
A) I only
B) II only
C) III only
D) I and II
E) All of these
(Hint:
Try to give them each a name.)

I.

II.

III.

A) I only
B) II only
C) III only
D) I and II
E) All of these

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
34
What is the name of the following branching alkyl group?
(The squiggly line represents a long straight chain.)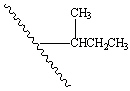
A) isopropyl
B) n-butyl
C) isobutyl
D) sec -butyl
E) tert -butyl
(The squiggly line represents a long straight chain.)

A) isopropyl
B) n-butyl
C) isobutyl
D) sec -butyl
E) tert -butyl

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
35
What is the IUPAC name for the compound with the following line-angle drawing?
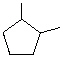
A) Methylcyclopentane
B) 1,2-dimethylcyclopentane
C) 1,2-dimethylpentane
D) 1,2-dimethylcyclohexane
E) 3,4-dimethylcyclohexane

A) Methylcyclopentane
B) 1,2-dimethylcyclopentane
C) 1,2-dimethylpentane
D) 1,2-dimethylcyclohexane
E) 3,4-dimethylcyclohexane

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
36
How are the following two compounds related?
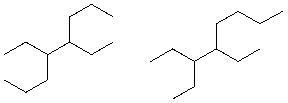
A) They are the same compound.
B) They are structural isomers.
C) They are cis - trans isomers.
D) They are completely different compounds.
E) They are chiral isomers.

A) They are the same compound.
B) They are structural isomers.
C) They are cis - trans isomers.
D) They are completely different compounds.
E) They are chiral isomers.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
37
What can be stated about the name, 4-Methylpentane?
(Hint:
Draw the molecule from the name given and then try to rename it.)
A) 4-Methylpentane is a valid name for a compound.
B) This name is wrong because the longest chain is six carbon atoms in length. It should be named Hexane.
C) This name is wrong because it does not provide the lowest number for the substituent "Methyl" group. It should be named 2-Methylpentane.
D) This name is wrong because the substituent group is "isopropyl" and the compound name should be 1-Isopropylpropane.
E) This name is wrong because it doesn't take into account that there are two methyl substituents at the branch point and the compound should be named 1,1-Dimethylbutane.
(Hint:
Draw the molecule from the name given and then try to rename it.)
A) 4-Methylpentane is a valid name for a compound.
B) This name is wrong because the longest chain is six carbon atoms in length. It should be named Hexane.
C) This name is wrong because it does not provide the lowest number for the substituent "Methyl" group. It should be named 2-Methylpentane.
D) This name is wrong because the substituent group is "isopropyl" and the compound name should be 1-Isopropylpropane.
E) This name is wrong because it doesn't take into account that there are two methyl substituents at the branch point and the compound should be named 1,1-Dimethylbutane.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
38
Compare compounds I-III with compound A. Which of the three compounds listed below is(are) the same compound as compound A?
(Hint:
Try to give them each a name.) A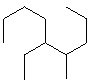
I.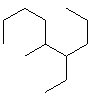
II.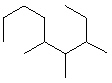
III.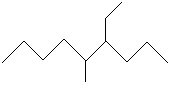
A) I only
B) II only
C) III only
D) I and II
E) I and III
(Hint:
Try to give them each a name.) A

I.

II.

III.

A) I only
B) II only
C) III only
D) I and II
E) I and III

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
39
What can be stated about the name of 1,1,1-Trimethylhexane?
(Hint:
Draw the molecule from the name given and then try to rename it.)
A) This name is a valid name for a compound.
B) This name is incorrect because it provides the wrong numbering scheme. The compound should be named 1,2,2-Trimethylhexane.
C) This name is incorrect because the longest chain is seven carbon atoms long and there are two substituent groups. The name of the compound should be 2,2-Dimethylheptane.
D) This name is incorrect because the longest chain is eight carbon atoms and there is one substituent groups. The name of the compound should be 2-Methyloctane.
E) This name is incorrect. There are nine carbon atoms and therefore the compound should be named Nonane.
(Hint:
Draw the molecule from the name given and then try to rename it.)
A) This name is a valid name for a compound.
B) This name is incorrect because it provides the wrong numbering scheme. The compound should be named 1,2,2-Trimethylhexane.
C) This name is incorrect because the longest chain is seven carbon atoms long and there are two substituent groups. The name of the compound should be 2,2-Dimethylheptane.
D) This name is incorrect because the longest chain is eight carbon atoms and there is one substituent groups. The name of the compound should be 2-Methyloctane.
E) This name is incorrect. There are nine carbon atoms and therefore the compound should be named Nonane.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
40
Name the compound:
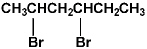
A) 1,3-dibromohexane
B) 2,4-dibromohexane
C) 3,5-dibromohexane
D) dibromohexane
E) none of these

A) 1,3-dibromohexane
B) 2,4-dibromohexane
C) 3,5-dibromohexane
D) dibromohexane
E) none of these

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
41
What is the correct IUPAC name for the following cyclic alkane?
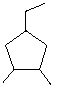
A) Cyclononane
B) 1-Ethyl-3,4-dimethylcyclopentane
C) 4-Ethyl-1,2-dimethylcyclopentane
D) 1,2-Dimethyl-4-ethylcyclohexane
E) 3-Ethyl-1,5-dimethylcyclopentane

A) Cyclononane
B) 1-Ethyl-3,4-dimethylcyclopentane
C) 4-Ethyl-1,2-dimethylcyclopentane
D) 1,2-Dimethyl-4-ethylcyclohexane
E) 3-Ethyl-1,5-dimethylcyclopentane

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
42
How are the following two compounds related?
A) They are the same compound.
B) They are structural isomers.
C) They are cis - trans isomers.
D) They are completely different compounds.
E) They are chiral isomers.
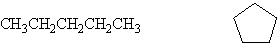
A) They are the same compound.
B) They are structural isomers.
C) They are cis - trans isomers.
D) They are completely different compounds.
E) They are chiral isomers.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
43
What product(s) is(are) formed when methane, CH4, is treated with chlorine and the mixture is exposed to light?
CH4 + Cl2→??
A) CH3Cl
B) CH2Cl2
C) CHCl3
D) CCl4
E) all of these
CH4 + Cl2→??
A) CH3Cl
B) CH2Cl2
C) CHCl3
D) CCl4
E) all of these

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
44
What is the correct IUPAC name for the following alkene?
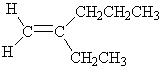
A) Ethylpropylethene
B) 2-Propyl-1-butene
C) 2-Ethyl-1-pentene
D) 3-Methylhexene
E) Heptene

A) Ethylpropylethene
B) 2-Propyl-1-butene
C) 2-Ethyl-1-pentene
D) 3-Methylhexene
E) Heptene

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
45
What is the correct IUPAC name for the following alkene?
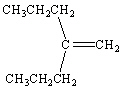
A) 4-methyl-4-heptene
B) 4-octene
C) 4-propyl-4-pentene
D) 2-propyl-1-pentene
E) 1,1-dipropylethene

A) 4-methyl-4-heptene
B) 4-octene
C) 4-propyl-4-pentene
D) 2-propyl-1-pentene
E) 1,1-dipropylethene

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
46
What is the correct IUPAC name for the following alkene?
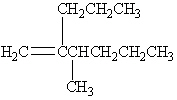
A) 4,5-Dimethyl-4-octene
B) 2-Butyl-3-Methyl-1-pentene
C) 3-Methyl-2-propyl-1-hexene
D) 2,3-Dipropyl-1-Butene
E) 3,4-Dipropyl-1-Butene

A) 4,5-Dimethyl-4-octene
B) 2-Butyl-3-Methyl-1-pentene
C) 3-Methyl-2-propyl-1-hexene
D) 2,3-Dipropyl-1-Butene
E) 3,4-Dipropyl-1-Butene

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
47
What is(are) the major product(s) of the following combustion reaction ?

A)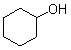
B)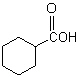
C)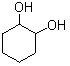
D)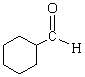
E) CO2 and H2O

A)

B)

C)

D)

E) CO2 and H2O

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
48
What is the systematic IUPAC name for the following cycloalkane?
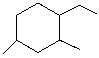
A) Cyclodecane
B) 1-Ethyl-2,4-Dimethylcyclohexane
C) 4-Ethyl-1,3-Dimethylcyclohexane
D) 2,4-Diethyl-1-propylcyclohexane
E) 1,3-Diethyl-4-propylcyclohexane

A) Cyclodecane
B) 1-Ethyl-2,4-Dimethylcyclohexane
C) 4-Ethyl-1,3-Dimethylcyclohexane
D) 2,4-Diethyl-1-propylcyclohexane
E) 1,3-Diethyl-4-propylcyclohexane

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
49
What are the products of the complete combustion of ethane?
CH3CH3 + O2→?
A) CO2 + H2O
B) CH3CO2H + H2
C) C + H2O + O2
D) CO2 + H2
E) CH3CH2OH
CH3CH3 + O2→?
A) CO2 + H2O
B) CH3CO2H + H2
C) C + H2O + O2
D) CO2 + H2
E) CH3CH2OH

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
50
How are the following two compounds related?
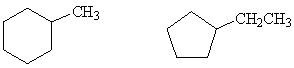
A) They are completely different compounds with different formulas.
B) They are structural isomers.
C) They are stereoisomers.
D) They are cis / trans isomers.
E) They are chiral isomers.
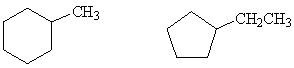
A) They are completely different compounds with different formulas.
B) They are structural isomers.
C) They are stereoisomers.
D) They are cis / trans isomers.
E) They are chiral isomers.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
51
What is the correct structure for the compound, 1,2-Diethylcyclohexane?
A)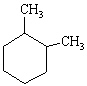
B)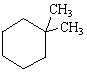
C)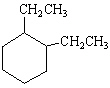
D)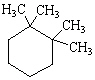
E)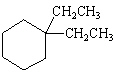
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
52
What is the correct IUPAC name for the following alkene?
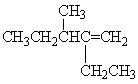
A) 2-ethyl-3-methyl-2-hexene
B) 2-ethyl-3-methyl-1-pentene
C) 4-ethyl-3-methyl-4-pentene
D) 3-methyl-4-ethyl-4-pentene
E) Octene

A) 2-ethyl-3-methyl-2-hexene
B) 2-ethyl-3-methyl-1-pentene
C) 4-ethyl-3-methyl-4-pentene
D) 3-methyl-4-ethyl-4-pentene
E) Octene

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
53
Which molecule from the list below is an alkene ?
A)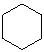
B)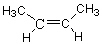
C)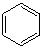
D)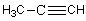
E)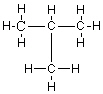
A)

B)
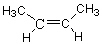
C)

D)
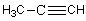
E)


Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
54
What is the correct IUPAC name for the following alkene?
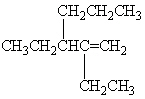
A) 3-Decene
B) 2-Ethyl-3-Propyl-1-Pentene
C) 4-Ethyl-3-Propyl-4-Pentene
D) 3-Ethyl-3-Heptene
E) 2,3-Diethyl-1-Hexene

A) 3-Decene
B) 2-Ethyl-3-Propyl-1-Pentene
C) 4-Ethyl-3-Propyl-4-Pentene
D) 3-Ethyl-3-Heptene
E) 2,3-Diethyl-1-Hexene

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
55
What is the correct IUPAC name for the following cycloalkane?
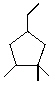
A) 1-Ethyl-3,3,4-trimethylcyclopentane
B) 1-Ethyl-3,4,4-trimethylcyclopentane
C) 1-Ethyl-3-isopropyl-4-methylcyclopentane
D) 4-Ethyl-1,1,2-trimethylcyclopentane
E) 4-Ethyl-1,2,2-trimethylcyclopentane

A) 1-Ethyl-3,3,4-trimethylcyclopentane
B) 1-Ethyl-3,4,4-trimethylcyclopentane
C) 1-Ethyl-3-isopropyl-4-methylcyclopentane
D) 4-Ethyl-1,1,2-trimethylcyclopentane
E) 4-Ethyl-1,2,2-trimethylcyclopentane

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
56
The molecular formula for butene is:
A) C4H4
B) C4H6
C) C4H8
D) C4H10
E) none of these
A) C4H4
B) C4H6
C) C4H8
D) C4H10
E) none of these

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
57
How are the following two compounds related?
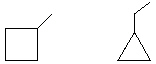
A) Completely different compounds
B) Identical compounds
C) Structural isomers
D) Cis - Trans isomers
E) Chiral isomers
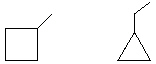
A) Completely different compounds
B) Identical compounds
C) Structural isomers
D) Cis - Trans isomers
E) Chiral isomers

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following hydrocarbons burns in the presence of excess oxygen to produce carbon dioxide, CO2, and water, H2O?
A) Methane
B) Ethene
C) Ethyne
D) Benzene
E) All of these
A) Methane
B) Ethene
C) Ethyne
D) Benzene
E) All of these

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
59
How are the following two compounds related?
A) Completely different compounds
B) Structural isomers
C) Cis - Trans isomers
D) Same compound
E) Chiral isomers

A) Completely different compounds
B) Structural isomers
C) Cis - Trans isomers
D) Same compound
E) Chiral isomers

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
60
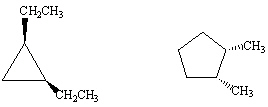
What is the IUPAC name and configuration for the following alkene?
A) cis -Decene
B) cis -4-Ethyl-5-Methyl-4-heptene
C) trans -4-Ethyl-5-Methyl-4-heptene
D) cis -4-Ethyl-3-Methyl-3-heptene
E) trans -4-Ethyl-3-Methyl-3-heptene

A) cis -Decene
B) cis -4-Ethyl-5-Methyl-4-heptene
C) trans -4-Ethyl-5-Methyl-4-heptene
D) cis -4-Ethyl-3-Methyl-3-heptene
E) trans -4-Ethyl-3-Methyl-3-heptene

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
61
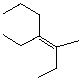
Starting from five carbons in a row as shown below in the skeletal structure, how many different alkenes (structural isomers) with a formula of C 5 H 10 are possible?
A) zero
B) one
C) two
D) three
E) four

A) zero
B) one
C) two
D) three
E) four

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
62
Which alkene listed below is a trans stereoisomer?
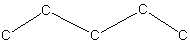
A)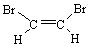
B)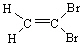
C)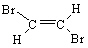
D)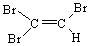
E)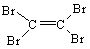
A)
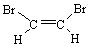
B)
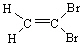
C)
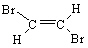
D)
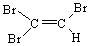
E)
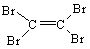

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following alkenes can have cis and trans isomers?
I. 1-propene
II. 2-butene
III. 3-hexene
A) I only
B) II only
C) III only
D) I and II
E) II and III
I. 1-propene
II. 2-butene
III. 3-hexene
A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
64
What is the correct IUPAC name for the following alkene?
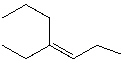
A) cis -3-propyl-3-hexene
B) trans -3-propyl-3-hexene
C) cis -4-Ethyl-4-heptene
D) cis -4-Ethyl-3-heptene
E) trans -4-Ethyl-3-heptene

A) cis -3-propyl-3-hexene
B) trans -3-propyl-3-hexene
C) cis -4-Ethyl-4-heptene
D) cis -4-Ethyl-3-heptene
E) trans -4-Ethyl-3-heptene

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
65
For which of the following can exhibit cis-trans isomerism possible?
I. 1,4-dichlorobenzene
II. 2,4-dichloro-2-butene
III. 1,1-dichloro-1-butene
A) I only
B) II only
C) III only
D) I and II
E) II and III
I. 1,4-dichlorobenzene
II. 2,4-dichloro-2-butene
III. 1,1-dichloro-1-butene
A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
66
From the list below, which compound is cis -2-hexene?
A)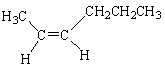
B)
C)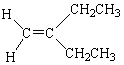
D)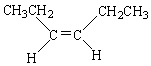
E)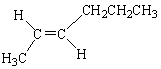
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
67
Which alkene listed below can have both cis and trans isomers?
I. 1-pentene
II. 2-pentene
III. 2-methyl-2-pentene
A) I only
B) II only
C) III only
D) I and II
E) II and III
I. 1-pentene
II. 2-pentene
III. 2-methyl-2-pentene
A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following alkenes can exhibit cis / trans isomerism?
I. 4-methyl-2-pentene
II. 3-methyl-2-pentene
III. 2-methyl-2-pentene
A) I only
B) I and II
C) I and III
D) II and III
E) All of these
I. 4-methyl-2-pentene
II. 3-methyl-2-pentene
III. 2-methyl-2-pentene
A) I only
B) I and II
C) I and III
D) II and III
E) All of these

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following alkenes can exhibit cis / trans isomerism?
I. 2-methyl-2-butene
II. 2-butene
III. 1-butene
A) I only
B) II only
C) III only
D) I and II
E) II and III
I. 2-methyl-2-butene
II. 2-butene
III. 1-butene
A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following molecules is Cis -3-hexene?
A)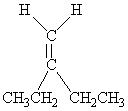
B)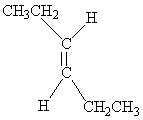
C)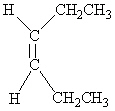
D)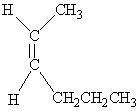
E)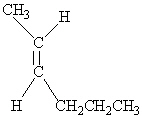
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following is Trans -3-Methyl-2-pentene?
A)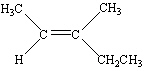
B)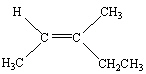
C)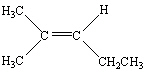
D)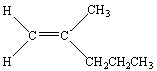
E)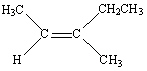
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
72
What is the correct IUPAC name for the following cycloalkene?
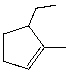
A) 1-Ethyl-2-methylcyclopentene
B) 2-Ethyl-1-methylcyclopentene
C) 2-Methyl-3-Ethylcyclopentene
D) 1-Ethyl-2-methylcyclooctene
E) 5-Ethyl-1-methylcyclopentene

A) 1-Ethyl-2-methylcyclopentene
B) 2-Ethyl-1-methylcyclopentene
C) 2-Methyl-3-Ethylcyclopentene
D) 1-Ethyl-2-methylcyclooctene
E) 5-Ethyl-1-methylcyclopentene

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following alkenes can have cis and trans isomers?
I. 2-methyl-2-hexene
II. 3-methyl-2-hexene
III. 2-methyl-3-hexene
A) I only
B) II only
C) III only
D) I and II
E) II and III
I. 2-methyl-2-hexene
II. 3-methyl-2-hexene
III. 2-methyl-3-hexene
A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
74
What is the IUPAC name for the following compound?
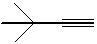
A) 2,2-dimethylpropyne
B) 3,3-dimethyl-1-butyne
C) 2,2-dimethyl-3-butyne
D) Hexyne
E) Tertbutylethyne
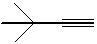
A) 2,2-dimethylpropyne
B) 3,3-dimethyl-1-butyne
C) 2,2-dimethyl-3-butyne
D) Hexyne
E) Tertbutylethyne

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following bonds allow(s) for free rotation?
I. A carbon-carbon single bond in an alkane
II. A carbon-carbon double bond in an alkene
III. A carbon-carbon single bond in a cyclic alkane
A) I only
B) II only
C) III only
D) I and II
E) I and III
I. A carbon-carbon single bond in an alkane
II. A carbon-carbon double bond in an alkene
III. A carbon-carbon single bond in a cyclic alkane
A) I only
B) II only
C) III only
D) I and II
E) I and III

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
76
Which compound listed below has a cis and trans isomer?
I. CH2=CCl2
II. CH3CCl=CCl2
III. CH3CCl=C(CH3)Cl
A) I and II, but not III
B) I and III, but not II
C) II and III, but not I
D) all three have cis and trans isomers
E) only III
I. CH2=CCl2
II. CH3CCl=CCl2
III. CH3CCl=C(CH3)Cl
A) I and II, but not III
B) I and III, but not II
C) II and III, but not I
D) all three have cis and trans isomers
E) only III

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is cis -4-Ethyl-3-heptene?
A)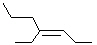
B)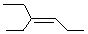
C)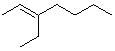
D)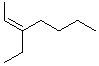
E)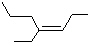
A)
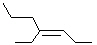
B)
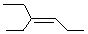
C)

D)

E)
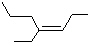

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
78
What is the correct IUPAC name for the following cyclic alkene?
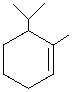
A) 1,2,2-Trimethylcyclohexene
B) 2-Methyl-1-propylcyclopentene
C) 1-Methyl-2-propylcyclohexene
D) 1-Isopropyl-2-methylcyclopentene
E) 6-Isopropyl-1-methylcyclohexene

A) 1,2,2-Trimethylcyclohexene
B) 2-Methyl-1-propylcyclopentene
C) 1-Methyl-2-propylcyclohexene
D) 1-Isopropyl-2-methylcyclopentene
E) 6-Isopropyl-1-methylcyclohexene

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following molecules is Trans -3-hexene?
A)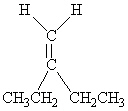
B)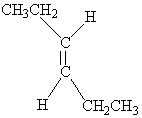
C)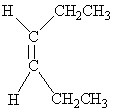
D)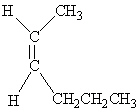
E)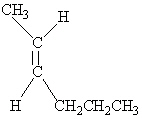
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following molecules is considered a cis alkene isomer?
A)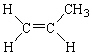
B)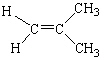
C)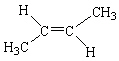
D)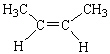
E)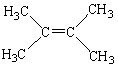
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck


