Deck 5: Solution Concentration
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/77
Play
Full screen (f)
Deck 5: Solution Concentration
1
There is a 9 M aqueous HCl solution in the stock room, but a 5 M solution is required for an experiment. Doubling the volume of the 9 M sample with water will produce the 5 M solution.
True
2
Solution concentration is an intensive property.
True
3
The following equation can be used when C represents either a M or % (w/v) concentration. 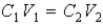
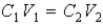
True
4
The conversion factor for converting from moles of HPO42-- to equivalents is: 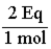
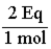

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
5
For a 2.00 M solution, the conversion factor for determining the number of moles of solute in a given volume of solution is: 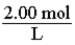

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
6
The %(v/v) of a solution can be defined as: 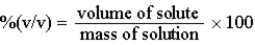

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
7
The solubility of a compound in water was measured and found to be 0.7 g/L. This compound would be classified as insoluble.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
8
Dialysis and osmosis are used for the same purposes.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
9
The concentration of cholesterol in plasma was determined to be 215 mg/dL. The mass of cholesterol in 59.1 mL of this plasma is 127 mg.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
10
In cases of cerebral endema, a hypertonic solution is administered. The goal of giving the patient the hypertonic solution would be to pull fluid from the cells through the process of osmosis.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
11
The total molarity of an intravenous solution is given on the label as 151 mEq/L. The osmolarity of this solution is also 151 mEq/L.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
12
Solution contains 55 mg of magnesium in 2.5 L of solution. The concentration of this solution is 2.2 mg/dL.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
13
A normal saline solution is a 0.90% (w/v) aqueous solution of NaCl. This is the same as: 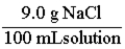
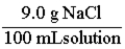

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
14
Putting a celery stick in distilled water results in the uptake of water by the celery.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
15
The solubility of Na2SO4 will probably increase with increasing temperature.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
16
Consider the two containers shown below separated by a seminpermeable membrane. When allowed to stand overnight,  water will have moved from the right-hand container to the left hand container..
water will have moved from the right-hand container to the left hand container..
 water will have moved from the right-hand container to the left hand container..
water will have moved from the right-hand container to the left hand container..
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
17
When you have your blood drawn, the most common method of expressing the plasma level of sodium and potassium is as mEq/L.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
18
The osmotic pressure of a 0.10 M NaCl solution will be the same as that of a 0.10 M urea solution.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
19
If you have 9 g of NaCl in 1 L of water, this solution is 0.9% NaCl and is called normal saline or NS.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
20
Consider the following graph. 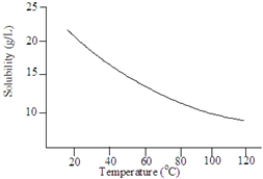 The solute is probably a gas.
The solute is probably a gas.
 The solute is probably a gas.
The solute is probably a gas.
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
21
What is the molar mass of ibuprofen, C13H18O2?
A) 29.0 g/mol
B) 206.3 g/mol
C) 289.4 g/mol
D) 377.7 g/mol
A) 29.0 g/mol
B) 206.3 g/mol
C) 289.4 g/mol
D) 377.7 g/mol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
22
Saline solutions (NaCl in water) used to deliver intravenous drugs are 0.89%(w/v). What mass of NaCl would be needed to prepare 450.0 mL of such a solution?
A)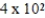 g
g
B) 0.89 g
C) 4.0 g
D) 5.1 g
A)
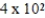 g
gB) 0.89 g
C) 4.0 g
D) 5.1 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
23
How many grams of solid KCl are needed to prepare 250.0 mL of 0.235 M solution?
A) 9.32 g
B) 31.3 g
C) 15.6 g
D) 4.38 g
A) 9.32 g
B) 31.3 g
C) 15.6 g
D) 4.38 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
24
A solution contains 35.5 mg of Vitamin C in 175 mL of solution. The concentration of this solution could be expressed as:
A) 203 ppm.
B) 0.0203% (m/v).
C) 20.3 mg/dL.
D) All of the above could be used.
A) 203 ppm.
B) 0.0203% (m/v).
C) 20.3 mg/dL.
D) All of the above could be used.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
25
What is the total molarity of solute particles in a 0.250 M solution of (NH4)2SO4?
A) 0.250 M
B) 0.500 M
C) 0.750 M
D) 0.0833 M
A) 0.250 M
B) 0.500 M
C) 0.750 M
D) 0.0833 M

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
26
Calculate the number of moles of ZnCl2, in 180.0 mL of 0.330 M solution.
A)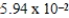 mol
mol
B) 1.83 mol
C) 0.545 mol
D) 59.4 mol
A)
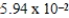 mol
molB) 1.83 mol
C) 0.545 mol
D) 59.4 mol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
27
A solution is made by dissolving 5.84 grams of NaCl in enough distilled water to give a final volume of 1.00 L. What is the molarity of the solution?
A) 0.100 M
B) 1.00 M
C) 0.0250 M
D) 0.400 M
A) 0.100 M
B) 1.00 M
C) 0.0250 M
D) 0.400 M

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
28
What is the molarity of a solution containing 0.585 mol of lactic acid in 250.0 mL of solution?
A) 146 M
B) 2.34 M
C) 0.427 M
D) 0.146 M
A) 146 M
B) 2.34 M
C) 0.427 M
D) 0.146 M

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
29
Calculate the total molarity of solute particles in a solution that contains 0.050 M glucose (a nonelectrolyte) and 0.200 M CaCl2.
A) 0.250 M
B) 0.450 M
C) 0.650 M
D) 0.350 M
A) 0.250 M
B) 0.450 M
C) 0.650 M
D) 0.350 M

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
30
How would the following solution be classified? 0.05 M in KCl and 0.14 M in glucose
A) isotonic
B) hypertonic
C) hypotonic
A) isotonic
B) hypertonic
C) hypotonic

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
31
A solution is made by dissolving 22.5 mL of oil in enough gasoline to give 60.5 mL of solution. What is the % (v/v) of oil in the solution?
A) 37.2 % (v/v)
B) 269% (v/v)
C) 0.372% (v/v)
D) 27.1% (v/v)
A) 37.2 % (v/v)
B) 269% (v/v)
C) 0.372% (v/v)
D) 27.1% (v/v)

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
32
A solution has a concentration of 16 ppm. Which of the following is another way to describe the concentration of this solution?
A) 0.016 ppb
B) 0.16 ppb
C)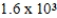 ppb
ppb
D)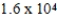 ppb
ppb
A) 0.016 ppb
B) 0.16 ppb
C)
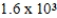 ppb
ppbD)
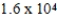 ppb
ppb
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following pass through both osmotic and dialysis membranes?
A) solvent molecules
B) large molecules
C) small molecules that are larger than solvent molecules
D) both solvent and small molecules
A) solvent molecules
B) large molecules
C) small molecules that are larger than solvent molecules
D) both solvent and small molecules

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the two containers shown below separated by a seminpermeable membrane. When allowed to stand overnight,  there will be no change in the liquid levels.
there will be no change in the liquid levels.
 there will be no change in the liquid levels.
there will be no change in the liquid levels.
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
35
The following list gives the concentration of the major components of blood plasma. Solute 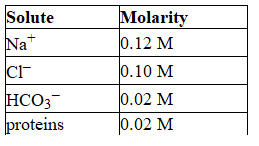 What is the total concentration of all the minor components in the plasma?
What is the total concentration of all the minor components in the plasma?
A) 0.02 M
B) 0.28 M
C) 0.12 M
D) Cannot be determined with the given information.
 What is the total concentration of all the minor components in the plasma?
What is the total concentration of all the minor components in the plasma?A) 0.02 M
B) 0.28 M
C) 0.12 M
D) Cannot be determined with the given information.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
36
Lactated Ringer's solution 109 mEq/L of Cl-. What is the mass of the chloride ion in 225 mL of this solution?
A) 24.5 g
B) 0.0245 g
C) 0.869 g
D) 0.109 g
A) 24.5 g
B) 0.0245 g
C) 0.869 g
D) 0.109 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is associated with cells in a hypertonic solution?
A) crenation
B) hemolysis
C) reverse osmosis
D) none of these
A) crenation
B) hemolysis
C) reverse osmosis
D) none of these

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
38
If 1.00 mol of each of the following solutes is dissolved in 2.00 L of an aqueous solution, which solution contains the largest number of solute particles?
A) LiBr
B) sucrose
C) Ca(NO3)2
D) Na3PO4
A) LiBr
B) sucrose
C) Ca(NO3)2
D) Na3PO4

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
39
What is the mass of salt in a 400.0 gram-sample of salt water which is 1.50% (w/w) salt?
A) 1.50 g
B) 2.67 g
C) 3.00 g
D) 6.00 g
A) 1.50 g
B) 2.67 g
C) 3.00 g
D) 6.00 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
40
What will happen to a red blood cell if placed in the following solution? 0.14 M lactose (a nonelectrolyte)
A) crenation
B) hemolysis
C) nothing
A) crenation
B) hemolysis
C) nothing

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
41
Consider two solutions, A and B, separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below. 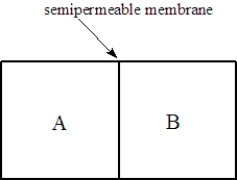 Fill the blank(s) with the appropriate terms from the list below.
Fill the blank(s) with the appropriate terms from the list below.
right
left
osmosis
dialysis
Solution A is 0.50 M in sucrose and Solution is 1.5 M in sucrose. After one hour, the compartment on the ____________________ will have the higher osmotic pressure.
 Fill the blank(s) with the appropriate terms from the list below.
Fill the blank(s) with the appropriate terms from the list below.right
left
osmosis
dialysis
Solution A is 0.50 M in sucrose and Solution is 1.5 M in sucrose. After one hour, the compartment on the ____________________ will have the higher osmotic pressure.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the solutions shown in the containers below. The composition of each solution is given in the image. 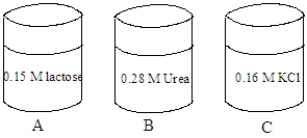 Fill the blank with the appropriate term from the list below.
Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
A red blood cell is placed in the solution in container A. The cell will undergo _____________________.
 Fill the blank with the appropriate term from the list below.
Fill the blank with the appropriate term from the list below. isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
A red blood cell is placed in the solution in container A. The cell will undergo _____________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
43
Based on the following graph, approximately what minimum temperature would be needed to dissolved 20 g of this solute in 1.00 L of water? 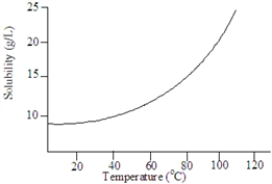
A) 9 C
B) 100 C
C) 35 C
D) 80 C

A) 9 C
B) 100 C
C) 35 C
D) 80 C

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
44
An oral rehydration solution contains 30 mEq/L of citrate3-, what is the molarity of citrate in this solution?
A) 0.09 M
B) 90 M
C) 10 M.
D) 0.01 M
A) 0.09 M
B) 90 M
C) 10 M.
D) 0.01 M

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
45
Consider the solutions shown in the containers below. The composition of each solution is given in the image.  Fill the blank with the appropriate term from the list below.
Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
A red blood cell is placed in the solution in container C. The cell will undergo ____________________.
 Fill the blank with the appropriate term from the list below.
Fill the blank with the appropriate term from the list below. isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
A red blood cell is placed in the solution in container C. The cell will undergo ____________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
46
Consider two solutions, A and B, separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below.  Fill the blank(s) with the appropriate terms from the list below.
Fill the blank(s) with the appropriate terms from the list below.
right
left
osmosis
dialysis
If the seminpermeable membrane has pores too small to allow glucose to pass through, ______________will not occur.
 Fill the blank(s) with the appropriate terms from the list below.
Fill the blank(s) with the appropriate terms from the list below.right
left
osmosis
dialysis
If the seminpermeable membrane has pores too small to allow glucose to pass through, ______________will not occur.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
47
Consider two solutions, A and B, separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below.  Fill the blank(s) with the appropriate terms from the list below.
Fill the blank(s) with the appropriate terms from the list below.
right
left
osmosis
dialysis
Solution A is pure water, and solution B is 0.05 M glucose. Water molecules will move to the ______________________compartment and glucose molecules will move to the __________________compartment.
 Fill the blank(s) with the appropriate terms from the list below.
Fill the blank(s) with the appropriate terms from the list below.right
left
osmosis
dialysis
Solution A is pure water, and solution B is 0.05 M glucose. Water molecules will move to the ______________________compartment and glucose molecules will move to the __________________compartment.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
48
Consider two solutions, A and B, separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below.  Fill the blank(s) with the appropriate terms from the list below.
Fill the blank(s) with the appropriate terms from the list below.
right
left
osmosis
dialysis
Solution A is 0.10 M in fructose and 0.05 M in glucose. Solution B is 0.20 M in sucrose. The direction of osmosis will be to the _______________________.
 Fill the blank(s) with the appropriate terms from the list below.
Fill the blank(s) with the appropriate terms from the list below.right
left
osmosis
dialysis
Solution A is 0.10 M in fructose and 0.05 M in glucose. Solution B is 0.20 M in sucrose. The direction of osmosis will be to the _______________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
49
A stockroom attendant has a 15.0% (w/v) solution of KOH. What volume of this solution should she use if she needs to prepare 20.0 mL of a 10.0% (w/v) solution?
A) 6.67 mL
B) 7.50 mL
C) 13.3 mL
D) 15.0 mL
A) 6.67 mL
B) 7.50 mL
C) 13.3 mL
D) 15.0 mL

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
50
If a solution contains 3.25 mol of Al3+, how many equivalents of Al3+ are present?
A) 3.25 Eq
B) 1.08 Eq
C) 6.50 Eq
D) 9.75 Eq
A) 3.25 Eq
B) 1.08 Eq
C) 6.50 Eq
D) 9.75 Eq

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
51
Consider two solutions, A and B, separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below.  Fill the blank(s) with the appropriate terms from the list below.
Fill the blank(s) with the appropriate terms from the list below.
right
left
osmosis
dialysis
Solution A is 0.10 M in lactose and 0.050 M in urea. Solution B is 0.15 M in KCl. Water will flow to the ______________________compartment.
 Fill the blank(s) with the appropriate terms from the list below.
Fill the blank(s) with the appropriate terms from the list below.right
left
osmosis
dialysis
Solution A is 0.10 M in lactose and 0.050 M in urea. Solution B is 0.15 M in KCl. Water will flow to the ______________________compartment.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
52
A normal concentration of sodium (Na) in blood plasma is 141 mEq/L (US average range is 135-145 mEq/L). How many mg of sodium are there in a 10.0 mL sample of his blood plasma?
A) 141 mg
B) 14.1 mg
C) 1.41 mg
D) 1.40 × 103 mg
A) 141 mg
B) 14.1 mg
C) 1.41 mg
D) 1.40 × 103 mg

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
53
Consider two solutions, A and B, separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below.  Fill the blank(s) with the appropriate terms from the list below.
Fill the blank(s) with the appropriate terms from the list below.
right
left
osmosis
dialysis
Solution A is 0.010 M glucose, and solution B is 0.050 M glucose. The glucose will dialyze to the _____________________.
 Fill the blank(s) with the appropriate terms from the list below.
Fill the blank(s) with the appropriate terms from the list below.right
left
osmosis
dialysis
Solution A is 0.010 M glucose, and solution B is 0.050 M glucose. The glucose will dialyze to the _____________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
54
How many mL of 6.00 M HCl are needed to prepare 1.50 L of 0.200 M HCl solution?
A) 1.80 × 104 mL
B) 125 mL
C) 2.00 × 10−3 mL
D) 50.0 mL
A) 1.80 × 104 mL
B) 125 mL
C) 2.00 × 10−3 mL
D) 50.0 mL

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
55
A solution contains 5.75 mg of magnesium ions in 332 mL of solution. What is the concentration of the solution in mEq/L?
A) 0.712 mEq/L
B) 0.473 mEq/L
C) 1.42 mEq/L.
D) 34.6 mEq/L
A) 0.712 mEq/L
B) 0.473 mEq/L
C) 1.42 mEq/L.
D) 34.6 mEq/L

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is(are) an example(s) of a hypertonic intravenous solution? (NS represents normal saline.)
A) 0.9% NS
B) 0.45% NS with D5 (termed half Normal Saline with Dextrose 5%)
C) a. D5 ½ NS (Dextrose 5% with half NS)
D) a. 2% NS
E) all are hypertonic solutions
F) only b and d are hypertonic solutions
A) 0.9% NS
B) 0.45% NS with D5 (termed half Normal Saline with Dextrose 5%)
C) a. D5 ½ NS (Dextrose 5% with half NS)
D) a. 2% NS
E) all are hypertonic solutions
F) only b and d are hypertonic solutions

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
57
A solution is prepared by adding 25.0 mL of 1.30 M glucose solution to a flask, and then adding enough water to give a final volume of 200.0 mL. What is the molarity of the solution?
A) 0.260 M
B) 0.163 M
C) 6.50 M
D) 1.24 M
A) 0.260 M
B) 0.163 M
C) 6.50 M
D) 1.24 M

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
58
Based on the following graph, the solubility of this substance at 80 °C is approximately: 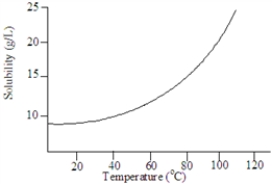
A) 25 g/L.
B) 15 g/L.
C) 10 g/L
D) 20 g/L

A) 25 g/L.
B) 15 g/L.
C) 10 g/L
D) 20 g/L

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
59
Consider the solutions shown in the containers below. The composition of each solution is given in the image.  Fill the blank with the appropriate term from the list below.
Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
A red blood cell is placed in the solution in container B. The cell will undergo ____________________.
 Fill the blank with the appropriate term from the list below.
Fill the blank with the appropriate term from the list below. isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
A red blood cell is placed in the solution in container B. The cell will undergo ____________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
60
A student is preparing a sugar water solution to make rock candy. When the student adds sugar to the solution, the added sugar does not dissolve. Which kind of solution does the student have?
A) a saturated solution
B) a solution at its solubility limit
C) an unsaturated solution
D) a suspension
A) a saturated solution
B) a solution at its solubility limit
C) an unsaturated solution
D) a suspension

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
61
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right, left, or no movement.
Answer the following questions as appropriate with: right, left, or no movement.
What is the total molarity of a solution containing 25.7 g of sucrose (C12H22O11) and 16.1 g of ribose (C5H10O5) dissolved in enough water to produce 850.0 mL of solution?
 Answer the following questions as appropriate with: right, left, or no movement.
Answer the following questions as appropriate with: right, left, or no movement.What is the total molarity of a solution containing 25.7 g of sucrose (C12H22O11) and 16.1 g of ribose (C5H10O5) dissolved in enough water to produce 850.0 mL of solution?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
62
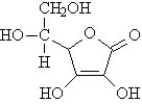
The vitamins A (retinol) and C (ascorbic acid) are shown below. All atoms other than C and H are explicitly shown. 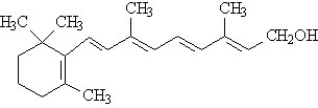
 A C
A C
Complete the following questions be entered in the appropriate letter (A or C) in the blank provided.
The beaker below contains oil (a compound of mostly carbon and hydrogen) in the upper layer and water in the lower layer. Oil floats on top of the water because it is less dense. The vitamin that would be the most soluble in the upper layer is ______.
The vitamin that would be the most soluble in the upper layer is ______.

 A C
A CComplete the following questions be entered in the appropriate letter (A or C) in the blank provided.
The beaker below contains oil (a compound of mostly carbon and hydrogen) in the upper layer and water in the lower layer. Oil floats on top of the water because it is less dense.
 The vitamin that would be the most soluble in the upper layer is ______.
The vitamin that would be the most soluble in the upper layer is ______.
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
63
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right, left, or no movement.
Answer the following questions as appropriate with: right, left, or no movement.
Upon standing the water molecules will move in which direction?
 Answer the following questions as appropriate with: right, left, or no movement.
Answer the following questions as appropriate with: right, left, or no movement.Upon standing the water molecules will move in which direction?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the following structure. 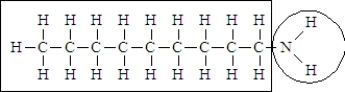 Complete the sentence using the appropriate terms given below.
Complete the sentence using the appropriate terms given below.
hydrophobic
hydrophilic
soluble
insoluble
The portion of the molecule in the box is classified as ______________________ and that in the circle is classified as_____________________.
 Complete the sentence using the appropriate terms given below.
Complete the sentence using the appropriate terms given below. hydrophobic
hydrophilic
soluble
insoluble
The portion of the molecule in the box is classified as ______________________ and that in the circle is classified as_____________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
65
The vitamins A (retinol) and C (ascorbic acid) are shown below. All atoms other than C and H are explicitly shown. 
 A C
A C
Complete the following questions be entered in the appropriate letter (A or C) in the blank provided.
The vitamin that would be classified as fat-soluble is ______.

 A C
A CComplete the following questions be entered in the appropriate letter (A or C) in the blank provided.
The vitamin that would be classified as fat-soluble is ______.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
66
The vitamins A (retinol) and C (ascorbic acid) are shown below. All atoms other than C and H are explicitly shown. 
 A C
A C
Complete the following questions be entered in the appropriate letter (A or C) in the blank provided.
Write the conversion factor that corresponds to 7.75%(w/v) NaCl.

 A C
A CComplete the following questions be entered in the appropriate letter (A or C) in the blank provided.
Write the conversion factor that corresponds to 7.75%(w/v) NaCl.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
67
The vitamins A (retinol) and C (ascorbic acid) are shown below. All atoms other than C and H are explicitly shown. 
 A C
A C
Complete the following questions be entered in the appropriate letter (A or C) in the blank provided.
The vitamin that would not be stored by the human body is ______.

 A C
A CComplete the following questions be entered in the appropriate letter (A or C) in the blank provided.
The vitamin that would not be stored by the human body is ______.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
68
The vitamins A (retinol) and C (ascorbic acid) are shown below. All atoms other than C and H are explicitly shown. 
 A C
A C
Complete the following questions be entered in the appropriate letter (A or C) in the blank provided.
The vitamin that would be the most hydrophobic is ______.

 A C
A CComplete the following questions be entered in the appropriate letter (A or C) in the blank provided.
The vitamin that would be the most hydrophobic is ______.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
69
For the following questions, fill in the blank with one of the following terms as appropriate.
increase
decrease
remains constant
cannot predict
As the size of the hydrophobic portion of a molecule increases, the solubility in water will ___________.
increase
decrease
remains constant
cannot predict
As the size of the hydrophobic portion of a molecule increases, the solubility in water will ___________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
70
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right, left, or no movement.
Answer the following questions as appropriate with: right, left, or no movement.
Calculate the percent concentration (m/v) of a solution prepared by dissolving 6.45 g of glucose in enough water to produce 85.0 mL of solution?
 Answer the following questions as appropriate with: right, left, or no movement.
Answer the following questions as appropriate with: right, left, or no movement.Calculate the percent concentration (m/v) of a solution prepared by dissolving 6.45 g of glucose in enough water to produce 85.0 mL of solution?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
71
For the following questions, fill in the blank with one of the following terms as appropriate.
increase
decrease
remains constant
cannot predict
For many ionic solids such as NaHCO3 water solubility will ___________ at higher temperatures..
increase
decrease
remains constant
cannot predict
For many ionic solids such as NaHCO3 water solubility will ___________ at higher temperatures..

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
72
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right, left, or no movement.
Answer the following questions as appropriate with: right, left, or no movement.
Upon standing the glucose molecules will move in which direction?
 Answer the following questions as appropriate with: right, left, or no movement.
Answer the following questions as appropriate with: right, left, or no movement.Upon standing the glucose molecules will move in which direction?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
73
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right, left, or no movement.
Answer the following questions as appropriate with: right, left, or no movement.
Calculate the parts per million (ppm) of a solution prepared by dissolving 7.45 mg of glucose in enough water to produce 145.0 mL of solution?
 Answer the following questions as appropriate with: right, left, or no movement.
Answer the following questions as appropriate with: right, left, or no movement.Calculate the parts per million (ppm) of a solution prepared by dissolving 7.45 mg of glucose in enough water to produce 145.0 mL of solution?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
74
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right, left, or no movement.
Answer the following questions as appropriate with: right, left, or no movement.
Upon standing the sucrose molecules will move in which direction?
 Answer the following questions as appropriate with: right, left, or no movement.
Answer the following questions as appropriate with: right, left, or no movement.Upon standing the sucrose molecules will move in which direction?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
75
For the following questions, fill in the blank with one of the following terms as appropriate.
increase
decrease
remains constant
cannot predict
When a beverage can is opened the pressure of the gas above the liquid will___________ causing the solubility of the gas to ____________.
increase
decrease
remains constant
cannot predict
When a beverage can is opened the pressure of the gas above the liquid will___________ causing the solubility of the gas to ____________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
76
Consider the solutions shown in the containers below. The composition of each solution is given in the image.  Fill the blank with the appropriate term from the list below.
Fill the blank with the appropriate term from the list below.
isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
The tonicity of the solution in container C is ______________________.
 Fill the blank with the appropriate term from the list below.
Fill the blank with the appropriate term from the list below. isotonic
hypotonic
hypertonic
crenation
hemolysis
no change
The tonicity of the solution in container C is ______________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
77
Consider the following structure.  Complete the sentence using the appropriate terms given below.
Complete the sentence using the appropriate terms given below.
hydrophobic
hydrophilic
soluble
insoluble
This molecule is probably____________________in water.
 Complete the sentence using the appropriate terms given below.
Complete the sentence using the appropriate terms given below. hydrophobic
hydrophilic
soluble
insoluble
This molecule is probably____________________in water.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck


