Deck 1: Measurements in Science and Medicine
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/77
Play
Full screen (f)
Deck 1: Measurements in Science and Medicine
1
An appropriate unit to measure the length of a football field would be the meter.
True
2
A 20.00 mL urine sample of a patient has a mass of 20.70
g. This patient is most likely drinking very large amounts of water.
g. This patient is most likely drinking very large amounts of water.
False
3
If the specific gravity of a sample of urine tested higher than normal, this would indicate dilution.
False
4
To convert feet to inches, you should multiply by the factor shown below. 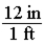
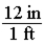

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
5
If a patient stands 6 feet tall, their height can also be expressed as 1828.8 cm.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
6
The average of the following volume measurements is 15.5 mL. 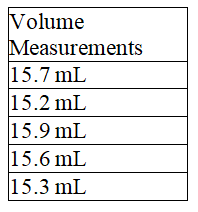


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
7
The lowest temperature ever recorded on earth was -128.6°F. The temperature is equivalent to - 89.2 Κ.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
8
The memory capacity of a flash drive is measured in gigabytes so that the capacity can be expressed using simple integers.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
9
The normal range (adult) for specific gravity of urine is 1.020 - 1.028 g/mL

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
10
Using a unit of mg to measure the mass of a premature infant would not be appropriate because the mass of the infant would be a very large number.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
11
To convert micrograms to grams, you should multiply by 1,000,000 g/μg.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
12
If an order read: NS (normal saline solution) 1000 mL to be given intravenously over 8 hrs. 125 mL of NS should be administered every hour.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
13
A Celsius degree is the same size as a Kelvin degree.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
14
Consider the image below.  The smallest division on the ruler is a cm.
The smallest division on the ruler is a cm.
 The smallest division on the ruler is a cm.
The smallest division on the ruler is a cm.
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
15
If the following represents a syringe that measures in cc's (cm3), the volume indicated by the end of the plunger would be correctly recorded as 5.2 cc. 


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
16
One advantage of the Kelvin system is that it is impossible to have temperatures below zero.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
17
The normal range (adult) for specific gravity of urine is 1.010 - 1.048 g/mL.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
18
A patient weights 220 lbs. A medication for this patient is supposed to be taken using a dosage of 3 mg per kg per day. The correct dose for this patient is 3000 mg per day.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
19
Consider the image showing a sample of modeling clay. 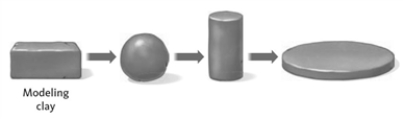 The density of the clay remains constant through the changes shown.
The density of the clay remains constant through the changes shown.
 The density of the clay remains constant through the changes shown.
The density of the clay remains constant through the changes shown.
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
20
A pharmaceutical solution of penicillin contains 125 mg of penicillin in 3 mL. The two conversion factors that express this relationship are: 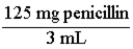 and
and 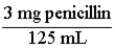
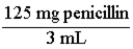 and
and 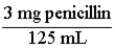

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following set-ups will allow you to calculate the cost of fruit in dollars per gram, if the price is given as 0.79 dollars per pound?
A)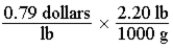
B)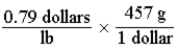
C)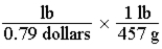
D)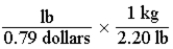
A)
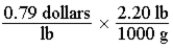
B)
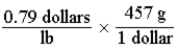
C)
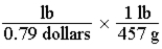
D)
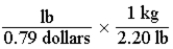

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
22
The following statement is correct based on the size of the measurement. "My cup of coffee contains 500 L."

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
23
A common piece of laboratory glassware is a 125 mL beaker. What is the volume of this piece of glassware in the English system of units? [1 quart = 0.946 L = 32 fl oz]
A) 0.423 fl oz
B) 0.423 quarts
C) 4.23 fl oz
D) 4.23 quarts
A) 0.423 fl oz
B) 0.423 quarts
C) 4.23 fl oz
D) 4.23 quarts

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
24
The prefix centi- denotes what fraction of a base unit?
A) 1/10
B) 1/100
C) 1/1000
D) 100
A) 1/10
B) 1/100
C) 1/1000
D) 100

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
25
An intern made an error and gave a patient a dose of 500 μg rather than 500 mg of a drug. Which of the following is true?
A) The patient received an overdose by a factor of 1000.
B) The patient received an overdose by a factor of 100.
C) The patient received an underdose by a factor of 1000.
D) The patient received an underdose by a factor of 100.
A) The patient received an overdose by a factor of 1000.
B) The patient received an overdose by a factor of 100.
C) The patient received an underdose by a factor of 1000.
D) The patient received an underdose by a factor of 100.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
26
The mass of an object is
A) the force between the object and the earth.
B) a measure of the amount of matter in the object.
C) the amount of space the object occupies.
D) depends on the location of the object on Earth.
A) the force between the object and the earth.
B) a measure of the amount of matter in the object.
C) the amount of space the object occupies.
D) depends on the location of the object on Earth.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
27
Aluminum has a density of 2.70 g/ mL. What volume is occupied by a block of aluminum that weighs 4.32 kg?
A) 0.000625 mL
B) 0.625 mL
C) 1.60 mL
D) 1.60 L
A) 0.000625 mL
B) 0.625 mL
C) 1.60 mL
D) 1.60 L

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
28
The base unit of length in the metric system is the
A) mil.
B) millimeter.
C) foot.
D) meter.
A) mil.
B) millimeter.
C) foot.
D) meter.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
29
A particular model of hybrid car can travel 53.0 miles/gallon of gas. What is this fuel efficiency expressed in the metric system? [1 quart = 0.946 L; 1 mile = 1.609 km]
A) 8.71 km/L
B) 20.2 km/L
C) 22.5 km/L
D) 90 km/L
A) 8.71 km/L
B) 20.2 km/L
C) 22.5 km/L
D) 90 km/L

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
30
The land surface area of the earth is approximately 1.49 × 108 km2. Which of the following is the correct way to write this in conventional notation?
A) 0.00000000149 km2
B) 149,000,000 km2
C) 14,900,000,000 km2
D) none of these
A) 0.00000000149 km2
B) 149,000,000 km2
C) 14,900,000,000 km2
D) none of these

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
31
The densities of the coinage metals (copper, silver and gold) are as follows: copper = 8.95 g/mL
Silver = 12.59 g/mL
Gold = 19.32 g/mL
A sample of material is found to have a mass if 33.03 grams, and have a volume of 2.624 mL. This is a sample of which of the coinage metals?
A) copper
B) silver
C) gold
D) It is not one of the coinage metals.
Silver = 12.59 g/mL
Gold = 19.32 g/mL
A sample of material is found to have a mass if 33.03 grams, and have a volume of 2.624 mL. This is a sample of which of the coinage metals?
A) copper
B) silver
C) gold
D) It is not one of the coinage metals.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
32
In which of the following are the masses given in the correct order?
A) cg > mg > g >kg
B) cg > g > kg > mg
C) kg > g > cg > mg
D) mg > cg > g > kg
A) cg > mg > g >kg
B) cg > g > kg > mg
C) kg > g > cg > mg
D) mg > cg > g > kg

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is the smallest number?
A) 5 × 103
B) 3 × 104
C) 2 × 10-5
D) 7 × 10-6
A) 5 × 103
B) 3 × 104
C) 2 × 10-5
D) 7 × 10-6

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
34
A penicillin derivative is used to treat infections with an adult 24-hour dosage of 35 mg/kg of body mass. This is to be given in three injections daily. This antibiotic is prepared by the pharmacy in solution form with a concentration of 130 mg/5mL. What volume in milliliters should be given in each injection to an adult with a mass of 12.5kg?
A) 5.6 mL
B) 17 mL
C) 50 mL
D) 0.32 mL
A) 5.6 mL
B) 17 mL
C) 50 mL
D) 0.32 mL

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
35
If urine has a density of 1.08 g/mL, what would be the mass of a 143 mL urine sample?
A) 154 g
B) 132 g
C) 143 g
D) 0.00699 g
A) 154 g
B) 132 g
C) 143 g
D) 0.00699 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
36
How many minutes are in a 30 day month? [Assume exactly 24 hours in a day]
A) 7.20 × 102 minutes
B) 4.32 × 104 minutes
C) 2.59 × 106 minutes
D) 3.11 × 107 minutes
A) 7.20 × 102 minutes
B) 4.32 × 104 minutes
C) 2.59 × 106 minutes
D) 3.11 × 107 minutes

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
37
A tablet contains 250 mg of penicillin while the solution form of the same antibiotic contains 250 mg of penicillin/5 mL. If a doctor was to prescribe that one-half of a scored tablet be taken four times a day, how many mL of the solution would be equivalent to this daily dosage?
A) 5.0 mL
B) 20. mL
C) 2.5 mL
D) 10. mL
A) 5.0 mL
B) 20. mL
C) 2.5 mL
D) 10. mL

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is true of the relationship between density and specific gravity?
A) They have different numerical values and different units.
B) They have nearly the same numerical value and the same units.
C) They have nearly the same numerical value but specific gravity is dimensionless.
D) They have the nearly same units but different numerical values.
A) They have different numerical values and different units.
B) They have nearly the same numerical value and the same units.
C) They have nearly the same numerical value but specific gravity is dimensionless.
D) They have the nearly same units but different numerical values.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is not a base unit of the metric system?
A) g
B) g/L
C) L
D) All are base units.
A) g
B) g/L
C) L
D) All are base units.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
40
In an experiment a solid sphere is placed in a cylinder containing the organic solvent cyclohexane (density = 0.778 g/mL).  Based on this picture, the sphere has a density:
Based on this picture, the sphere has a density:
A) greater than 0.778 g/mL
B) less than 0.778 g/mL
C) about the same as 0.778 g/mL
D) The image does not provide enough information to answer.
 Based on this picture, the sphere has a density:
Based on this picture, the sphere has a density:A) greater than 0.778 g/mL
B) less than 0.778 g/mL
C) about the same as 0.778 g/mL
D) The image does not provide enough information to answer.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
41
The thermostat on an incubator reads 65°C. What is this temperature on the Kelvin scale?
A) 338 K
B) 149 K
C) -208 K
D) 65 K
A) 338 K
B) 149 K
C) -208 K
D) 65 K

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
42
The following questions refer to the plastic box shown below. 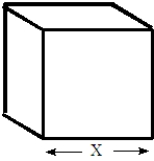 Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
dm
L
μL
g/mL
kg
μm
km
If a measurement were made of the quantity represented by X in the figure, an appropriate unit to use would be__________________________ if X = 18 in.
 Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.dm
L
μL
g/mL
kg
μm
km
If a measurement were made of the quantity represented by X in the figure, an appropriate unit to use would be__________________________ if X = 18 in.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
43
The boiling point of liquid nitrogen is 77 K. What is this temperature on the Celsius scale?
A) 350°C
B) 171°C
C) 25°C
D) -196°C
A) 350°C
B) 171°C
C) 25°C
D) -196°C

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
44
If a patient weighs 203 pounds (lbs), how many kilograms (kg) does the patient weigh?
A) 203 kg
B) 92.1 kg
C) 448 kg
D)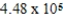 kg
kg
A) 203 kg
B) 92.1 kg
C) 448 kg
D)
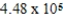 kg
kg
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
45
The following questions refer to the plastic box shown below.  Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
dm
L
μL
g/mL
kg
μm
km
The box is filled with water to the very top from a graduated cylinder. A unit that could be used to measure this quantity would be ______________________.
 Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.dm
L
μL
g/mL
kg
μm
km
The box is filled with water to the very top from a graduated cylinder. A unit that could be used to measure this quantity would be ______________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
46
The following questions refer to the plastic box shown below.  Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
dm
L
μL
g/mL
kg
μm
km
If the box were placed on a balance, a unit that might appear on the balance read-out would be _______________________.
 Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.dm
L
μL
g/mL
kg
μm
km
If the box were placed on a balance, a unit that might appear on the balance read-out would be _______________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
47
At what temperature do the temperatures on the Celsius and Kelvin scales have the same numerical value?
A) - 40
B) 0
C) 32
D) There is no value where the two scales are the same.
A) - 40
B) 0
C) 32
D) There is no value where the two scales are the same.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
48
If the density of ethanol is 0.787 g/mL, what is the mass of 37.4 mL of this substance?
A) 47.5 g
B) 29.4 g
C) 37.4 g
D) 0.0210 g
A) 47.5 g
B) 29.4 g
C) 37.4 g
D) 0.0210 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
49
In the health sciences, learning and understanding how to accurately convert chemical quantities is of utmost importance, as it keeps your patients and your practice safe. At home medicines are sometimes dispensed by the teaspoon (tsp) or tablespoon (tbsp). If there are 3 tsp in 1 tbsp and 1 tbsp is equal to 15 mL, how many milliliters are 2.0 tsp?
A) 45 mL
B) 45.0 mL
C) 20 mL
D) 3.0 mL
A) 45 mL
B) 45.0 mL
C) 20 mL
D) 3.0 mL

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
50
The following questions refer to the plastic box shown below.  Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
dm
L
μL
g/mL
kg
μm
km
Fill in the blanks with top or bottom as appropriate. In order to convert from kilograms to grams, the conversion factor should have 1 kg on the ____________________ and 1000 g on the _____________________.
 Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.dm
L
μL
g/mL
kg
μm
km
Fill in the blanks with top or bottom as appropriate. In order to convert from kilograms to grams, the conversion factor should have 1 kg on the ____________________ and 1000 g on the _____________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the two images below showing the readout on two balances. 
 A B Which balance should be able to produce more precise measurements?
A B Which balance should be able to produce more precise measurements?
A) A
B) B
C) The accuracy cannot be determined.

 A B Which balance should be able to produce more precise measurements?
A B Which balance should be able to produce more precise measurements?A) A
B) B
C) The accuracy cannot be determined.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
52
How many signficant figures are in the the measurement given below? 220.10 mm
A) 2
B) 3
C) 4
D) 5
A) 2
B) 3
C) 4
D) 5

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
53
The following questions refer to the plastic box shown below.  Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
dm
L
μL
g/mL
kg
μm
km
Fill in the first blank with the appropriate number (1, 2, 3 etc.) and the second blank with the direction (right or left). In order to convert from milliliters to liters, the decimal is moved ___________________ places to the___________________.
 Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.dm
L
μL
g/mL
kg
μm
km
Fill in the first blank with the appropriate number (1, 2, 3 etc.) and the second blank with the direction (right or left). In order to convert from milliliters to liters, the decimal is moved ___________________ places to the___________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
54
Compare the following two metric rulers. Fill in the blanks, respectively, with the identity of the ruler (A or B) and the terms more or less as appropriate. 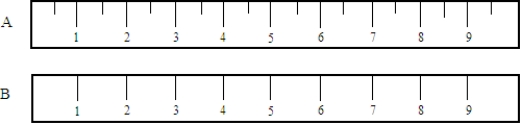 A measurement made with ruler_____________________would be ___________________accurate.
A measurement made with ruler_____________________would be ___________________accurate.
 A measurement made with ruler_____________________would be ___________________accurate.
A measurement made with ruler_____________________would be ___________________accurate.
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
55
Consider the image shown below.  Which of the following is an appropriate unit to place on this measurement?
Which of the following is an appropriate unit to place on this measurement?
A) g
B) mL
C) mm
D) cm3
 Which of the following is an appropriate unit to place on this measurement?
Which of the following is an appropriate unit to place on this measurement?A) g
B) mL
C) mm
D) cm3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
56
Based on the ruler represented in the picture. Use the appropriate integer (0, 1, 2, 3, etc.) in the blank to answer the question. 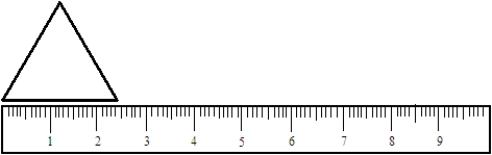 The length of the side of the triangle should be recorded to _____________________decimal places.
The length of the side of the triangle should be recorded to _____________________decimal places.
 The length of the side of the triangle should be recorded to _____________________decimal places.
The length of the side of the triangle should be recorded to _____________________decimal places.
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
57
The following questions refer to the plastic box shown below.  Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
dm
L
μL
g/mL
kg
μm
km
Fill in the first blank with the appropriate number (1, 2, 3, etc.) and the second blank with the direction (right or left). In order to convert from kilogram to milligrams, the decimal is moved ___________________ places to the___________________.
 Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.
Fill in the blanks in the questions from the following list. All units in the list will not be used and a unit maybe used more than once.dm
L
μL
g/mL
kg
μm
km
Fill in the first blank with the appropriate number (1, 2, 3, etc.) and the second blank with the direction (right or left). In order to convert from kilogram to milligrams, the decimal is moved ___________________ places to the___________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
58
Consider the two images below. 
 A B Which balance shows the more accurate measurement?
A B Which balance shows the more accurate measurement?
A) A
B) B
C) The accuracy cannot be determined.

 A B Which balance shows the more accurate measurement?
A B Which balance shows the more accurate measurement?A) A
B) B
C) The accuracy cannot be determined.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
59
How many millimeters are equivalent to 40.5 km?
A)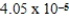 mm
mm
B)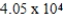 mm
mm
C)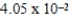 mm
mm
D)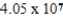 mm
mm
A)
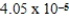 mm
mmB)
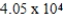 mm
mmC)
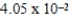 mm
mmD)
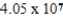 mm
mm
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
60
What temperature on the Celsius is the same as normal body temperature 98.6°F?
A) 34.3°C
B) 37.0°C
C) 119.9°C
D) none of these
A) 34.3°C
B) 37.0°C
C) 119.9°C
D) none of these

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
61
Fill in the blank with the appropriate number (0, 1 ,2 ,3 etc.). Consider the following calculation:
143.321 g
17.89 g
+ 100.1 g
261.311 g
The answer should be round to _____________________decimal places.
143.321 g
17.89 g
+ 100.1 g
261.311 g
The answer should be round to _____________________decimal places.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
62
Enter the number (0, 1 ,2, 3, etc.) in the blank provided. Consider the measurement shown below. 23.5410 g
The first uncertain digit is the _______________________.
The first uncertain digit is the _______________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
63
Enter the number (0, 1, 2, 3, etc.) in the blank provided. Consider the measurement shown below. 780 mg
The first uncertain digit is the _______________________.
The first uncertain digit is the _______________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
64
A patient is experiencing pain and you want to administer pain medication. The order reads Morphine 2 mg IV q (every) 4-6 hrs (hours) PRN (as needed) for pain. The pharmacy has supplied Morphine 10 mg/mL vials. How many mL's are to be administered to your patient?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
65
Fill in the blanks, respectively, with a letter (A or B) to represent the balance and more or less to describe the precision. Two students measured the density of a metal block was determined based on mass measurements using the same balance. The results are shown below. 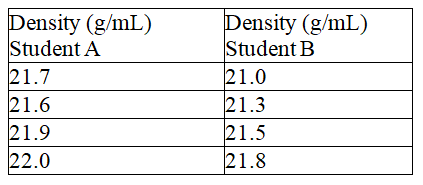 If the metal block is gold (density = 21.45 g/mL), student ___________________'s data is ____________________accurate.
If the metal block is gold (density = 21.45 g/mL), student ___________________'s data is ____________________accurate.
 If the metal block is gold (density = 21.45 g/mL), student ___________________'s data is ____________________accurate.
If the metal block is gold (density = 21.45 g/mL), student ___________________'s data is ____________________accurate.
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
66
In an ampule, Sublimaze 2 mL containing 50 mcg/mL is available. The order is to administer Sublimaze 0.05 mg intravenously (IV). (Note: mcg is another abbreviation for μg.)
a. How many mcg will you be giving?
b. How many mL will you be giving?
a. How many mcg will you be giving?
b. How many mL will you be giving?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
67
An angiotensin converting enzyme (ACE) inhibitor, from the dicarboxylate-containing agent group, Lisinopril, is ordered as an oral anti-hypertensive (blood pressure lowering) medication. The order reads, Lisinopril 50 mg po (by mouth) qd (each day). The pharmacy supplied Lisinopril 20 mg tablets. How many tablets would be needed each day?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
68
A patient arrives in the emergency department with a highly elevated blood pressure of 210/100 mm Hg. A calcium channel blocker and potent vasodilator is ordered. The entry of calcium into the cell causes the cell to contract, therefore a calcium channel blocker blocks the entry of calcium into the cell. This action causes decreased contraction, which in turn causes the vessels to relax and vasodilate, which in turn causes decreased blood pressure. The order reads diltiazem 15 mg IV stat. Diltiazem is supplied in 20 mg/2 mL vials. How many mL's would be administered?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
69
Fill in the blanks, respectively, with a letter (A or B) to represent the balance and more or less to describe the precision. The density of a metal block was determined based on mass measurements using two different balances. The results are shown below. 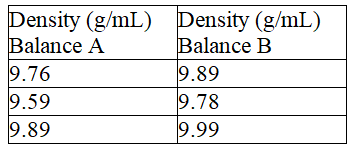 The density determined using balance ___________________ is ____________________precise.
The density determined using balance ___________________ is ____________________precise.
 The density determined using balance ___________________ is ____________________precise.
The density determined using balance ___________________ is ____________________precise.
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
70
Fill in the blank with the appropriate number (0, 1, 2, 3 etc.). The thermometer shown in the image is laying on a counter top and indicates room temperature.  This temperature contains _____________________significant figures..
This temperature contains _____________________significant figures..
 This temperature contains _____________________significant figures..
This temperature contains _____________________significant figures..
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
71
Write the complete name of the metric unit below in the blank. mm: ______________

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
72
Fill in the blanks, respectively, with higher or lower and adequate or inadequate. A drop of a potential donor's blood is placed in water and floats on the surface. This indicates that specific gravity of the blood is _______________________than water and that the iron concentration is___________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
73
The order reads 'haloperidol (generic name) 1 mg IV x1 now'. Haloperidol comes supplied in an ampule containing 10 mg/1 mL. How many mL's will you administer? Show your work.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
74
Digoxin is a purified cardiac glycoside, which is a commonly prescribed drug given to a patient experiencing a cardiac disorder, such as atrial fibrillation or atrial flutter. Digoxin 0.125 mg tablets are available. The order is for Digoxin 0.25 mg poq am (by mouth every morning). How many tablets will you give?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
75
Consider the metric railraod shown below. Fill in the blank with 10, 100, 1000, 10000, etc. as appropriate.  To move between three stops corresponds to increasing or decreasing the unit by a factor of _____________.
To move between three stops corresponds to increasing or decreasing the unit by a factor of _____________.
 To move between three stops corresponds to increasing or decreasing the unit by a factor of _____________.
To move between three stops corresponds to increasing or decreasing the unit by a factor of _____________.
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
76
Fill in the blank with the appropriate number (0, 1, 2, 3 etc.). The following calculation was carried out to determine the volume of a rectangular solid. 15.55 cm × 12.0 cm × 0.557 cm = 105.80233350 cm3
The answer should be round to _____________________significant figures..
The answer should be round to _____________________significant figures..

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
77
Fill in the blank with the appropriate term from the following: distance, volume, mass. Kilogram is a unit of _______________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck


