Deck 2: Atoms, Elements, and Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/77
Play
Full screen (f)
Deck 2: Atoms, Elements, and Compounds
1
An element has the following electron arrangement. shell 1: 2 electrons shell 2: 8 electrons shell 3: 18 electrons shell 4: 18 electrons shell 5: 7 electrons
The Lewis structure for this element would be: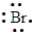
The Lewis structure for this element would be:
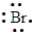
False
2
Sodium is a highly reactive metal and chlorine is a toxic gas, but when they come together the resulting material, sodium chloride (a white solid), is essential for life. Which of the following is true when sodium and chlorine are brought into contact with one another?
A) They form a heterogeneous mixture.
B) They form a homogenous mixture
C) They form a new element.
D) They form a compound.
A) They form a heterogeneous mixture.
B) They form a homogenous mixture
C) They form a new element.
D) They form a compound.
They form a compound.
3
If the atom ratio of Na to N in a compound is 3:1, the mole ratio of Na to N is 3:1.
True
4
The elements sodium, potassium, and oxygen are considered to be "elements of life" and are in the category of electrolytes.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
5
The atomic weight of phosphorus (P) is 30.91 amu or about 31 amu. This indicates that each P atom consists of 15 protons and 16 neutrons.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
6
In 22.99 g of Na there is 6.022 × 1023 atoms of Na.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
7
Electron shells define a region in space around the nucleus occupied by certain electrons.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
8
Group 4A and Group 14 are two different designations for the same column of the periodic table.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
9
The statement below describes an intensive property.  "The cost of this container of milk is $2.99/gal."
"The cost of this container of milk is $2.99/gal."
 "The cost of this container of milk is $2.99/gal."
"The cost of this container of milk is $2.99/gal."
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
10
An element is a shiny gray solid that can be pressed into a thin sheet. This element is probably a metalloid.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
11
One of the orbitals in a shell of an atom could be pictured as shown below. 


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
12
Elements in the same period of the periodic table generally show similar chemical behavior.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
13
When a particular solid sample is examined under a microscope, it is observed that there are regions that are black and regions which are yellow. What type of matter is this sample?
A) a compound
B) an element
C) a homogeneous mixture
D) a heterogeneous mixture
A) a compound
B) an element
C) a homogeneous mixture
D) a heterogeneous mixture

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
14
The pie chart shown below represents the elemental composition of the human body including water. 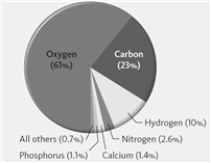


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
15
An elements with an electron arrangement of:
shell 1: 2 electrons shell 2: 8 electrons shell 3: 18 electrons shell 4: 18 electrons shell 5: 7 electrons
would have 1 valence electron.
shell 1: 2 electrons shell 2: 8 electrons shell 3: 18 electrons shell 4: 18 electrons shell 5: 7 electrons
would have 1 valence electron.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
16
An isotope of gallium consisting of 31 protons and 37 neutrons can be represented using the symbol shown below. gallium-37

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
17
The electron arrangement for Na would be:
shell 1: 2 electrons shell 2: 8 electrons shell 3: 1 electron
shell 1: 2 electrons shell 2: 8 electrons shell 3: 1 electron

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
18
A mole of an element contains the same number of atoms as a mole of any other element.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
19
Neutral isotopes of the same element have the same number of electrons.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
20
The alkali metal found in period 2 is lithium.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
21
Use the periodic table to determine about how many helium atoms (He) on the average would be needed to get close to the same mass as an oxygen atom (O).
A) 6
B) 4
C) 12
D) 1/4
A) 6
B) 4
C) 12
D) 1/4

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is true of the atomic weight of an element?
A) It is the weight of heaviest isotope.
B) It is the weight lightest isotope.
C) It is the weight of the most abundant isotope.
D) It is an average obtained from the weights and abundances of the isotopes.
A) It is the weight of heaviest isotope.
B) It is the weight lightest isotope.
C) It is the weight of the most abundant isotope.
D) It is an average obtained from the weights and abundances of the isotopes.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is the conversion factor that could be used to convert a mass in grams of sodium to the corresponding number of moles?
A)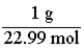
B)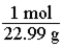
C)
D)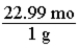
A)
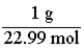
B)
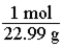
C)

D)
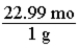

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
24
Naturally occurring lithium (Li) consists of only two isotopes, Li-6 (6.02 amu) and Li-7 (7.02 amu), where the exact isotopic masses are given in parentheses. Use the periodic table and determine which isotope is present in the larger percentage in the natural element.
A)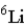
B)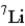
C) The percentage of each isotope is about the same.
D) The relative percent abundance cannot be determined from the information available.
A)
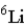
B)
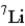
C) The percentage of each isotope is about the same.
D) The relative percent abundance cannot be determined from the information available.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
25
Convert the following number of moles into the corresponding mass. 5.22 mol Br
A) 15.3 g
B) 0.0653 g
C) 417 g
D) 79.9 g
A) 15.3 g
B) 0.0653 g
C) 417 g
D) 79.9 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
26
How many moles of sulfur are there in a 0.685-g sample of sulfur?
A) 0.0214 mol
B) 46.8 mol
C) 22.0 mol
D) 32.1 mol
A) 0.0214 mol
B) 46.8 mol
C) 22.0 mol
D) 32.1 mol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
27
The number of valence electrons of a representative element is related to which of the following?
A) atomic number
B) atomic weight
C) group number
D) period number
A) atomic number
B) atomic weight
C) group number
D) period number

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
28
What are the horizontal rows of the periodic table called?
A) cycles
B) periods
C) groups
D) columns
A) cycles
B) periods
C) groups
D) columns

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
29
A sealed cylinder is filled with a large collection of atoms that have 14 neutrons and 13 protons. Which of the following would behave in a manner similar to this collection of atoms?
A) a group of atoms with 13 neutrons and 14 protons
B) a group of atoms with 14 neutrons and 15 protons
C) a group of atoms with 15 neutrons and 14 protons
D) a group of atoms with 15 neutrons and 13 protons
A) a group of atoms with 13 neutrons and 14 protons
B) a group of atoms with 14 neutrons and 15 protons
C) a group of atoms with 15 neutrons and 14 protons
D) a group of atoms with 15 neutrons and 13 protons

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
30
What is the mass number of an atom that is made up of 38 protons, 52 neutrons and 38 electrons?
A) 38
B) 52
C) 90
D) 128
A) 38
B) 52
C) 90
D) 128

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following correctly describes a proton on a subatomic scale?
A) It is massive and has a +1 charge.
B) It is massive and has a -1 charge.
C) It has very small mass and a +1 charge.
D) It has a very small mass and a -1 charge.
A) It is massive and has a +1 charge.
B) It is massive and has a -1 charge.
C) It has very small mass and a +1 charge.
D) It has a very small mass and a -1 charge.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
32
What are the elements in the "A" columns of the period table called?
A) representative elements
B) nonmetal elements
C) metalloid elements
D) transition elements
A) representative elements
B) nonmetal elements
C) metalloid elements
D) transition elements

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following sequences gives the correct order as we move from left to right across a row of the period table?
A) metal, metalloid, nonmetal
B) metal, nonmetal, metalloid
C) nonmetal, metal, metalloid
D) nonmetal, metalloid, metal
A) metal, metalloid, nonmetal
B) metal, nonmetal, metalloid
C) nonmetal, metal, metalloid
D) nonmetal, metalloid, metal

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
34
Which column of the periodic table is commonly called the halogens?
A) 1A
B) 4A
C) 7A
D) 8A
A) 1A
B) 4A
C) 7A
D) 8A

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
35
How many valence electrons are there in an oxygen atom?
A) 2
B) 4
C) 6
D) 8
A) 2
B) 4
C) 6
D) 8

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is the Lewis structure for a nitrogen atom?
A)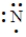
B)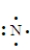
C)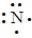
D)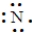
A)
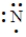
B)
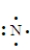
C)
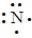
D)
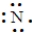

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
37
If 1 mol of an element has a mass of 63.54 g, the symbol for this element is
A) Eu.
B) Zn.
C) Cu.
D) Xe.
A) Eu.
B) Zn.
C) Cu.
D) Xe.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
38
Which subatomic particle(s) are found in the nucleus?
A) only electrons
B) only neutrons
C) only protons
D) both protons and neutrons
A) only electrons
B) only neutrons
C) only protons
D) both protons and neutrons

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following groups of elements contains only metals?
A) Ag, As, Ba, Ca
B) Ag, Au, Pb, Rb
C) As, Ge, Si, Te
D) B, Al, Ga, In
A) Ag, As, Ba, Ca
B) Ag, Au, Pb, Rb
C) As, Ge, Si, Te
D) B, Al, Ga, In

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is a chemical substance than can be broken down into another substance?
A) element
B) homogeneous mixture
C) heterogeneous mixture
D) compound
A) element
B) homogeneous mixture
C) heterogeneous mixture
D) compound

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
41
The image shown below represents an atomic view of a compound CsI.  What is the mass of one formula unit?
What is the mass of one formula unit?
A) 259.8 g
B) 259.8 amu
C) 1.00 g
D) 6.022 × 1023 amu
 What is the mass of one formula unit?
What is the mass of one formula unit?A) 259.8 g
B) 259.8 amu
C) 1.00 g
D) 6.022 × 1023 amu

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
42
Complete the following statement using one of the following terms.
compound
element
mixture
In the manufacture of steel, the percent of manganese is adjusted to determine the brittleness of the product. Steel is an example of a ______________________.
compound
element
mixture
In the manufacture of steel, the percent of manganese is adjusted to determine the brittleness of the product. Steel is an example of a ______________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
43
Use one the following terms to complete the given statement.
salt
sugar
vegetable oil
When ____________________ is added to water a heterogeneous mixture forms.
salt
sugar
vegetable oil
When ____________________ is added to water a heterogeneous mixture forms.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
44
Sodium chlorate, an ingredient in many common herbicides, has sodium, chlorine and oxygen atoms in the ratio 1:1:3, respectively. What is the formula unit for sodium chlorate?
A) NaCO3
B) SoClO3
C) NaClO3
D) none of these
A) NaCO3
B) SoClO3
C) NaClO3
D) none of these

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
45
A person drinks 1900 g of water, H2O, per day. How many moles of water did they consume?
A)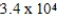 mol
mol
B) 0.009 mol
C) 105 mol
D) 18.02 mol
A)
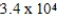 mol
molB) 0.009 mol
C) 105 mol
D) 18.02 mol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
46
What is the formula weight of ibuprofen, C13H18O2?
A) 29.0 g/mol
B) 206.3 g/mol
C) 289.4 g/mol
D) 377.7 g/mol
A) 29.0 g/mol
B) 206.3 g/mol
C) 289.4 g/mol
D) 377.7 g/mol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
47
Compound consists of five atoms of carbon, ten atoms of hydrogen, and five atoms of oxygen. The formula for this compound is
A) CH2O.
B) C10H5O10.
C) C5H10O5.
D) C5H10O10.
A) CH2O.
B) C10H5O10.
C) C5H10O5.
D) C5H10O10.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
48
What is the mass of one molecule of glucose, C6H12O6?
A) 29.02 amu
B) 180.2 amu
C) 29.02 g
D) 180.2 g
A) 29.02 amu
B) 180.2 amu
C) 29.02 g
D) 180.2 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
49
What is the mass of one mole of glucose, C6H12O6?
A) 29.02 amu
B) 180.2 amu
C) 29.02 g
D) 180.2 g
A) 29.02 amu
B) 180.2 amu
C) 29.02 g
D) 180.2 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
50
What is the meaning of the subscripts in the formula for ethyl alcohol, C2H5OH?
A) Each formula unit contains 2 carbon atoms for each oxygen atom.
B) There are two carbon atoms per formula unit of ethyl alcohol.
C) Each formula unit contains 3 times as many hydrogen atoms as carbon atoms.
D) All of these are correct statements.
A) Each formula unit contains 2 carbon atoms for each oxygen atom.
B) There are two carbon atoms per formula unit of ethyl alcohol.
C) Each formula unit contains 3 times as many hydrogen atoms as carbon atoms.
D) All of these are correct statements.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
51
What is the mass of 3.71 mol diethyl ether, C4H10O?
A) 20.0 g
B) 0.0501 g
C) 74.1 g
D) 275 g
A) 20.0 g
B) 0.0501 g
C) 74.1 g
D) 275 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
52
Use the following terms to complete the two statements given. A term may be used more than once.
heterogeneous mixture
homogeneous mixture
compound
In a restaurant fresh ground pepper is added to olive oil as a dipping sauce for bread. This dip represents a_____________________. Coffee with caramel flavoring is served for dessert. The coffee is an example of a _______________________.
heterogeneous mixture
homogeneous mixture
compound
In a restaurant fresh ground pepper is added to olive oil as a dipping sauce for bread. This dip represents a_____________________. Coffee with caramel flavoring is served for dessert. The coffee is an example of a _______________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following is the correct unit for the formula weight of a large number of atoms?
A) amu
B) g
C) mol
D) molecules
A) amu
B) g
C) mol
D) molecules

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
54
Enter the chemical symbol in the blank for the element that has the electron arrangement given below.
shell 1: 2 electrons shell 2: 8 electrons shell 3: 5 electrons
Symbol for the element:_____________________
shell 1: 2 electrons shell 2: 8 electrons shell 3: 5 electrons
Symbol for the element:_____________________

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
55
Use the following terms as appropriate to complete the given statement. All terms may not be used.
mass
volume
density
intensive
extensive
The ______________________ of a substance is an example of a ____________________ property.
mass
volume
density
intensive
extensive
The ______________________ of a substance is an example of a ____________________ property.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
56
If you need a sample of 2.841 mol of Na2S, how many grams do you need?
A) 0.003640 g
B) 2.841 g
C) 78.05 g
D) 221.7 g
A) 0.003640 g
B) 2.841 g
C) 78.05 g
D) 221.7 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
57
How many moles of oxygen would be required to make 4 mol of the following compound? P4O10
A) 40 mol O
B) 4 mol O
C) 16 mol O
D) 2.5 mol O
A) 40 mol O
B) 4 mol O
C) 16 mol O
D) 2.5 mol O

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
58
Enter the chemical symbol of the element in the blank. An element has the following electron arrangement. shell 1: 2 electrons shell 2: 8 electrons shell 3: 18 electrons shell 4: 18 electrons shell 5: 4 electrons
The reactivity of this element would most closely resemble that of which element?
Chemical symbol:_____________________
The reactivity of this element would most closely resemble that of which element?
Chemical symbol:_____________________

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
59
Enter the chemical symbol in the blank for the element described given below. Representative element in period 4 whose chemical behavior resembles that of O. Symbol for the element:_____________________

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
60
Consider the atomic view of a substance shown below.  This substance would be classified as a(n)
This substance would be classified as a(n)
A) element
B) compound
C) heterogeneous mixture
D) homogeneous mixture
 This substance would be classified as a(n)
This substance would be classified as a(n)A) element
B) compound
C) heterogeneous mixture
D) homogeneous mixture

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
61
Indicate the number of dots that should be placed around the Lewis symbol for the following element. Use an integer: 1, 2, 3, etc. as appropriate. Br

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
62
Write the chemical symbol for the elements that is in period 2 and group 8A of the periodic table.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
63
Based on the text periodic table and using the correct number of significant figures, enter the appropriate number in the blank. There are _______mol is 72.36 g of citric acid, C6H8O7.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
64
Enter an integer number (1, 2, 3, ...) in the blank. Sucrose (table sugar) has the formula C12H22O11. How many oxygen atoms would be in 3 formula units of sucrose?
____________________oxygen atoms.
____________________oxygen atoms.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
65
Based on the text periodic table and using the correct number of significant figures, enter the appropriate number in the blank. The mass of 5.00 mol of oxygen atoms is _____________g.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
66
Write the name of the following element. Sn

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
67
Enter the chemical symbol of the element in the blank. Calcium forms the compound CaF2 . What other alkaline earth element with a smaller atomic mass would form a compound with a similar formula?
Chemical symbol:_____________________.
Chemical symbol:_____________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
68
Fill in each blank with the appropriate term from the list given below.
protons
neutrons
electrons
valence electrons
14N and 15N
have the same number of _____________________.
protons
neutrons
electrons
valence electrons
14N and 15N
have the same number of _____________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
69
Fill in each blank with the appropriate term from the list given below.
protons
neutrons
electrons
valence electrons
14N and 15O have the same number of _____________________.
protons
neutrons
electrons
valence electrons
14N and 15O have the same number of _____________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
70
Based on the text periodic table and using the correct number of significant figures, enter the appropriate number in the blank. There are _______mol is 54.05 g of boron.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
71
Enter an integer number (1, 2, 3, ...) in the blank. How many electrons are in shell 2 of a P atom?
____________________electrons
____________________electrons

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
72
Based on the text periodic table and using the correct number of significant figures, enter the appropriate number in the blank. The mass of 7.00 mol of sodium chloride, NaCl, is _____________g.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
73
Classify the following statement as representing an intensive or extensive property by placing intensive or extensive in the blank. "Vinegar tastes sour.": _________________

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
74
Convert the following mass moles. 22.98 g glycine, an amino acid, C2H5NO2.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
75
Fill in each blank with the appropriate term from the list given below.
protons
neutrons
electrons
valence electrons
N, P and As have the same number of_____________________.
protons
neutrons
electrons
valence electrons
N, P and As have the same number of_____________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
76
Enter an integer number (1, 2, 3, ...) in the blank. Sucrose (table sugar) has the formula C12H22O11. A sample of sucrose contains 2400 carbon atoms. How many formula units does this represent?
____________________formula units.
____________________formula units.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
77
Enter the chemical symbol of the element in the blank. An element in period 3 has the Lewis structure: 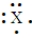 X should be replaced with what chemical symbol?
X should be replaced with what chemical symbol?
Chemical symbol:_____________________.
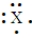 X should be replaced with what chemical symbol?
X should be replaced with what chemical symbol?Chemical symbol:_____________________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck


