Deck 16: Mixed Cultures
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/9
Play
Full screen (f)
Deck 16: Mixed Cultures
1
Organism A grows on substrate S and produces product P, which is the only substrate that or-
ganism B can utilize. The batch kinetics are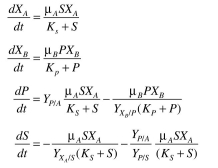 Assume the following parameter values:
Assume the following parameter values: 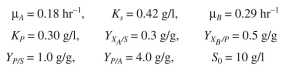 Determine the behavior of these two organisms in a chemostat. Plot S, P,
Determine the behavior of these two organisms in a chemostat. Plot S, P,  versus
versus
dilution rate. Discuss what happens to organism B as the dilution rate approaches the washout
dilution rate for organism A. (Courtesy of L. Erickson, from "Collected Coursework Prob-
lems in Biochemical Engineering," compiled by H. W. Blanch for 1977 Am. Soc. Eng. Educ.
Summer School.)
ganism B can utilize. The batch kinetics are
 Assume the following parameter values:
Assume the following parameter values: 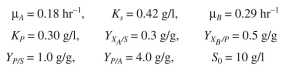 Determine the behavior of these two organisms in a chemostat. Plot S, P,
Determine the behavior of these two organisms in a chemostat. Plot S, P, 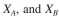 versus
versusdilution rate. Discuss what happens to organism B as the dilution rate approaches the washout
dilution rate for organism A. (Courtesy of L. Erickson, from "Collected Coursework Prob-
lems in Biochemical Engineering," compiled by H. W. Blanch for 1977 Am. Soc. Eng. Educ.
Summer School.)
Steady state material balances for a Chemostat with 2 Organisms and Commensalism  ……(1)
……(1)  …… (2)
…… (2) 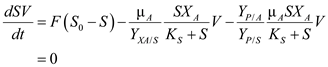 ……(3)
……(3) 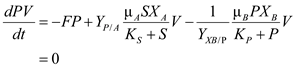 …… (4)
…… (4)
Solving equation (1) for D ( F/V ) as a function of S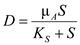 …… (5)
…… (5)
Solving equation (2) for P as a function of S and D …… (6)
…… (6)  …… (7)
…… (7)
Solving equation (3) for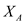 as a function of S , D and P
as a function of S , D and P 
 …… (8)
…… (8)
Solving equation (4) for as a function of S , D , P and
as a function of S , D , P and 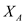


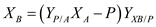 …… (9)
…… (9)
Choose S and use equation (5) to compute D , equation (7) to compute P , equation (8) to compute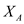 , and equation (9) to compute
, and equation (9) to compute 
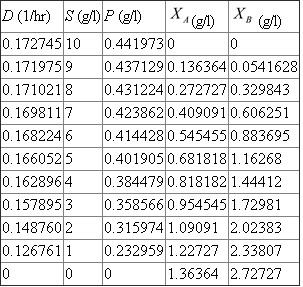
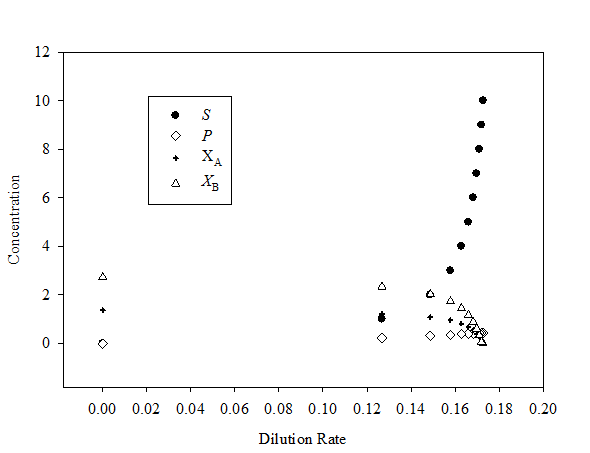 Since organism B relies on organism A to produce its substrate, P, washout of organism A, and thus the cessation of P production, results in washout of organism B as well. In examining equation (8) notice the direct dependence of
Since organism B relies on organism A to produce its substrate, P, washout of organism A, and thus the cessation of P production, results in washout of organism B as well. In examining equation (8) notice the direct dependence of  on
on 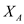
 ……(1)
……(1)  …… (2)
…… (2) 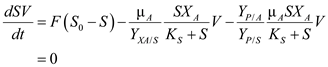 ……(3)
……(3) 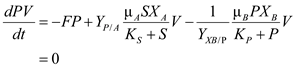 …… (4)
…… (4)Solving equation (1) for D ( F/V ) as a function of S
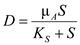 …… (5)
…… (5)Solving equation (2) for P as a function of S and D
 …… (6)
…… (6)  …… (7)
…… (7)Solving equation (3) for
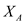 as a function of S , D and P
as a function of S , D and P 
 …… (8)
…… (8)Solving equation (4) for
 as a function of S , D , P and
as a function of S , D , P and 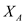


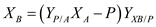 …… (9)
…… (9) Choose S and use equation (5) to compute D , equation (7) to compute P , equation (8) to compute
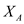 , and equation (9) to compute
, and equation (9) to compute 

 Since organism B relies on organism A to produce its substrate, P, washout of organism A, and thus the cessation of P production, results in washout of organism B as well. In examining equation (8) notice the direct dependence of
Since organism B relies on organism A to produce its substrate, P, washout of organism A, and thus the cessation of P production, results in washout of organism B as well. In examining equation (8) notice the direct dependence of  on
on 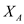
2
The 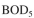 value of a waste-water feed stream to an activated-sludge unit is
value of a waste-water feed stream to an activated-sludge unit is 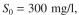 and the effluent is desired to be S = 30 mg/l. The feed flow rate is
and the effluent is desired to be S = 30 mg/l. The feed flow rate is 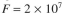 l/day. For the
l/day. For the
recycle ratio of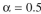 and a steady-state biomass concentration of X = 5 g/l, calculate the
and a steady-state biomass concentration of X = 5 g/l, calculate the
following:
a. Required reactor volume (V).
b. Biomass concentration in recycle
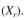
c. Solids (cells) residence time
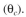
d. Hydraulic residence time
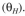
e. Determine the daily oxygen requirement.
Use the following kinetic parameters:
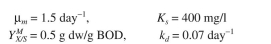
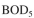 value of a waste-water feed stream to an activated-sludge unit is
value of a waste-water feed stream to an activated-sludge unit is 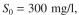 and the effluent is desired to be S = 30 mg/l. The feed flow rate is
and the effluent is desired to be S = 30 mg/l. The feed flow rate is 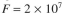 l/day. For the
l/day. For therecycle ratio of
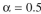 and a steady-state biomass concentration of X = 5 g/l, calculate the
and a steady-state biomass concentration of X = 5 g/l, calculate thefollowing:
a. Required reactor volume (V).
b. Biomass concentration in recycle
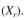
c. Solids (cells) residence time
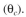
d. Hydraulic residence time
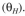
e. Determine the daily oxygen requirement.
Use the following kinetic parameters:
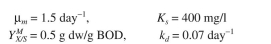
a) Required reactor volume ( V ) 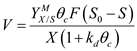

 b) Biomass concentration in recycle ( X r )
b) Biomass concentration in recycle ( X r ) 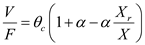
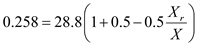

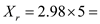 14.9 g/l
14.9 g/l
c) Solids (cells) residence time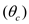

 d) Hydraulic residence time
d) Hydraulic residence time 
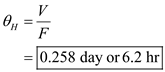 e) Determine the daily oxygen requirement.
e) Determine the daily oxygen requirement. 
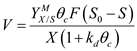

 b) Biomass concentration in recycle ( X r )
b) Biomass concentration in recycle ( X r ) 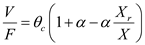
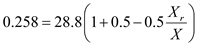

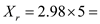 14.9 g/l
14.9 g/lc) Solids (cells) residence time
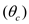

 d) Hydraulic residence time
d) Hydraulic residence time 
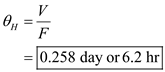 e) Determine the daily oxygen requirement.
e) Determine the daily oxygen requirement. 
3
For the activated-sludge unit shown in Fig. 16.7, the specific growth rate of cells is given by 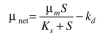 The following parameter values are known: F = 500 1/h,
The following parameter values are known: F = 500 1/h, 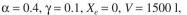
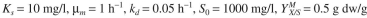 substrate.
substrate.
a. Calculate the substrate concentration (S) in the reactor at steady state.
b. Calculate the cell concentration(s) in the reactor.
c. Calculate Xr and Sr in the recycle stream.
Figure 16.7
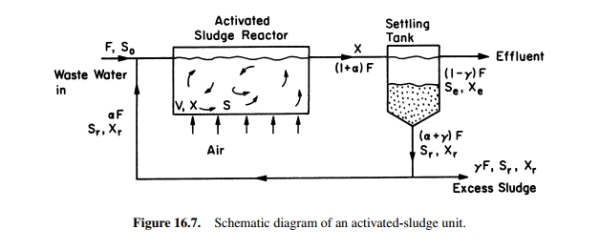
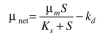 The following parameter values are known: F = 500 1/h,
The following parameter values are known: F = 500 1/h, 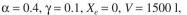
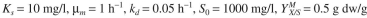 substrate.
substrate. a. Calculate the substrate concentration (S) in the reactor at steady state.
b. Calculate the cell concentration(s) in the reactor.
c. Calculate Xr and Sr in the recycle stream.
Figure 16.7

(a)The value of  is zero therefore the above expression of biomass material balance equation becomes,
is zero therefore the above expression of biomass material balance equation becomes,  Substitute tha values and solve,
Substitute tha values and solve,  Rarrange the ratio and solve,
Rarrange the ratio and solve,  Reduced biomass material balance equation (from equation16.35 provided in text book) is expressed as follows,
Reduced biomass material balance equation (from equation16.35 provided in text book) is expressed as follows, 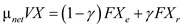 The value of the
The value of the  is zero, therefore the above expression becomes,
is zero, therefore the above expression becomes, 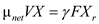 Rearrange the expression for
Rearrange the expression for  ,
, 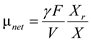 Substitute the value and solve,
Substitute the value and solve,  From the question provided in text book,
From the question provided in text book,  Substitute the value and solve for
Substitute the value and solve for 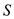 ,
, 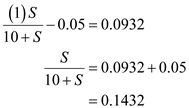 Further solve,
Further solve, 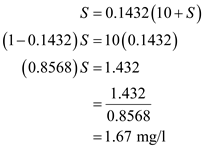 Therefore, substrate concentration
Therefore, substrate concentration 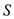 is
is  (b)The expression of the Cell concentartion in the reactor is,
(b)The expression of the Cell concentartion in the reactor is, 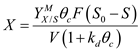 Now, the is calculated as follow,
Now, the is calculated as follow,  Substitude the values from part (a) and solve,
Substitude the values from part (a) and solve, 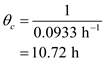 Substitute tha values and solve,
Substitute tha values and solve,  Hence the value of the cell concentration in the reactor is
Hence the value of the cell concentration in the reactor is 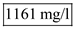 (c)
(c)
The value of the X r and S r in the recycle stream is calculated as follows,
The ratio is calculated in part (a) therefore rearrange this to find the value of
is calculated in part (a) therefore rearrange this to find the value of  ,
,  Rearrange for
Rearrange for  and then substitute the value of
and then substitute the value of 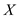 from above and solve,
from above and solve, 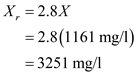 Hence the value of
Hence the value of  is
is 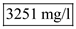 The value of
The value of  is same as
is same as 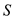 , therefore,
, therefore, 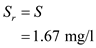 Hence the value of
Hence the value of  is
is 
 is zero therefore the above expression of biomass material balance equation becomes,
is zero therefore the above expression of biomass material balance equation becomes,  Substitute tha values and solve,
Substitute tha values and solve,  Rarrange the ratio and solve,
Rarrange the ratio and solve,  Reduced biomass material balance equation (from equation16.35 provided in text book) is expressed as follows,
Reduced biomass material balance equation (from equation16.35 provided in text book) is expressed as follows, 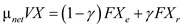 The value of the
The value of the  is zero, therefore the above expression becomes,
is zero, therefore the above expression becomes, 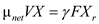 Rearrange the expression for
Rearrange the expression for  ,
, 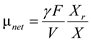 Substitute the value and solve,
Substitute the value and solve,  From the question provided in text book,
From the question provided in text book,  Substitute the value and solve for
Substitute the value and solve for 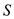 ,
, 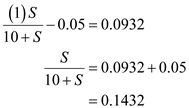 Further solve,
Further solve, 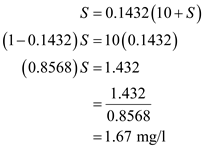 Therefore, substrate concentration
Therefore, substrate concentration 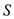 is
is  (b)The expression of the Cell concentartion in the reactor is,
(b)The expression of the Cell concentartion in the reactor is, 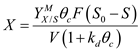 Now, the is calculated as follow,
Now, the is calculated as follow,  Substitude the values from part (a) and solve,
Substitude the values from part (a) and solve, 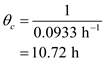 Substitute tha values and solve,
Substitute tha values and solve,  Hence the value of the cell concentration in the reactor is
Hence the value of the cell concentration in the reactor is 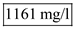 (c)
(c)The value of the X r and S r in the recycle stream is calculated as follows,
The ratio
 is calculated in part (a) therefore rearrange this to find the value of
is calculated in part (a) therefore rearrange this to find the value of  ,
,  Rearrange for
Rearrange for  and then substitute the value of
and then substitute the value of 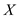 from above and solve,
from above and solve, 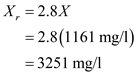 Hence the value of
Hence the value of  is
is 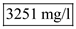 The value of
The value of  is same as
is same as 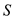 , therefore,
, therefore, 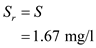 Hence the value of
Hence the value of  is
is 
4
In a trickling biological filter, the BOD value of the feed stream is 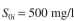 with a feed
with a feed
flow of F =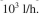 The effluent BOD value is desired to be
The effluent BOD value is desired to be 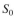 = 10 mg/l. The following ki-
= 10 mg/l. The following ki-
netic parameters for the biocatalysts are known: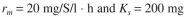 S/l. The
S/l. The
biofilm thickness is L = 0.1 mm. The cross-sectional area of the filter is A = 2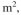 and the biofilm surface area per unit volume of the bed is
and the biofilm surface area per unit volume of the bed is 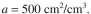 Assume that dis-
Assume that dis-
solved oxygen is the rate-limiting substrate and the diffusion coefficient of oxygen is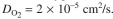 Determine the required height of the bed. You can assume first-order
Determine the required height of the bed. You can assume first-order
bioreaction kinetics.
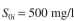 with a feed
with a feedflow of F =
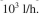 The effluent BOD value is desired to be
The effluent BOD value is desired to be 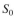 = 10 mg/l. The following ki-
= 10 mg/l. The following ki-netic parameters for the biocatalysts are known:
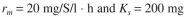 S/l. The
S/l. Thebiofilm thickness is L = 0.1 mm. The cross-sectional area of the filter is A = 2
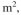 and the biofilm surface area per unit volume of the bed is
and the biofilm surface area per unit volume of the bed is 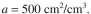 Assume that dis-
Assume that dis-solved oxygen is the rate-limiting substrate and the diffusion coefficient of oxygen is
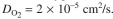 Determine the required height of the bed. You can assume first-order
Determine the required height of the bed. You can assume first-orderbioreaction kinetics.

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
5
An activated-sludge waste treatment system is required to reduce the amount of 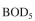 from
from
1000 mg/l to 20 mg/l at the exit. The sedimentation unit concentrates biomass by a factor of
3. Kinetic parameters are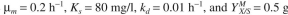 MLVSS/g
MLVSS/g 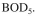 The flow of waste water is 10000 l/h and the size of the treatment basin is 50,000 l.
The flow of waste water is 10000 l/h and the size of the treatment basin is 50,000 l.
a. What is the value of the solids residence time
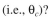
b. What value of the recycle ratio must be used?
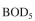 from
from1000 mg/l to 20 mg/l at the exit. The sedimentation unit concentrates biomass by a factor of
3. Kinetic parameters are
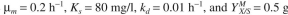 MLVSS/g
MLVSS/g 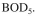 The flow of waste water is 10000 l/h and the size of the treatment basin is 50,000 l.
The flow of waste water is 10000 l/h and the size of the treatment basin is 50,000 l. a. What is the value of the solids residence time
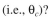
b. What value of the recycle ratio must be used?

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
6
Consider a well-mixed waste treatment system for a small-scale system. The system is oper-
ated with a reactor of 1000 l and flow rate of 100 l/h. The separator concentrates biomass by
a factor of 2. The recycle ratio is 0.7. The kinetic parameters are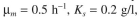
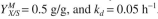 What is the exit substrate concentration?
What is the exit substrate concentration?
ated with a reactor of 1000 l and flow rate of 100 l/h. The separator concentrates biomass by
a factor of 2. The recycle ratio is 0.7. The kinetic parameters are
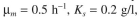
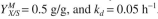 What is the exit substrate concentration?
What is the exit substrate concentration?
Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
7
Redo Example 16.4 if the Contois equation for growth applies. In this case 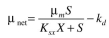 The values of
The values of  are the same as for Example 16.4, but
are the same as for Example 16.4, but 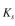 no longer applies. Assume
no longer applies. Assume 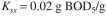 MLVSS.
MLVSS.
Example 16.4.
An industrial waste with an inlet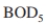 of 800 mg/l must be treated to reduce the exit
of 800 mg/l must be treated to reduce the exit 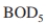 level to 20 mg/l. The inlet flow rate is 400 m ³/h. Kinetic parameters have been estimated for waste as
level to 20 mg/l. The inlet flow rate is 400 m ³/h. Kinetic parameters have been estimated for waste as 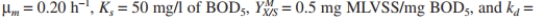
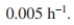 A waste treatment unit of 3200 m ³is available. Assume a recycle ratio of 0.40 and
A waste treatment unit of 3200 m ³is available. Assume a recycle ratio of 0.40 and 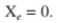 If you operate at a value of c 120 h, find S and determine if sufficient
If you operate at a value of c 120 h, find S and determine if sufficient 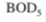 removal is attained in a well-mixed activated-sludge process to meet specifications. What will
removal is attained in a well-mixed activated-sludge process to meet specifications. What will
be X and the sludge production rate from this process?
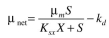 The values of
The values of  are the same as for Example 16.4, but
are the same as for Example 16.4, but 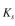 no longer applies. Assume
no longer applies. Assume 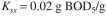 MLVSS.
MLVSS.Example 16.4.
An industrial waste with an inlet
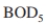 of 800 mg/l must be treated to reduce the exit
of 800 mg/l must be treated to reduce the exit 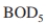 level to 20 mg/l. The inlet flow rate is 400 m ³/h. Kinetic parameters have been estimated for waste as
level to 20 mg/l. The inlet flow rate is 400 m ³/h. Kinetic parameters have been estimated for waste as 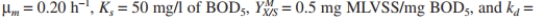
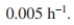 A waste treatment unit of 3200 m ³is available. Assume a recycle ratio of 0.40 and
A waste treatment unit of 3200 m ³is available. Assume a recycle ratio of 0.40 and 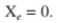 If you operate at a value of c 120 h, find S and determine if sufficient
If you operate at a value of c 120 h, find S and determine if sufficient 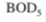 removal is attained in a well-mixed activated-sludge process to meet specifications. What will
removal is attained in a well-mixed activated-sludge process to meet specifications. What willbe X and the sludge production rate from this process?

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
8
A batch fermenter receives 1 l of medium with 5 g/l of glucose, which is the growth-rate-
limiting nutrient for a mixed population of two bacteria (a strain of E. coli and Azotobacter
vinelandii). A. vinelandii is five times larger than E. coli. The replication rates for the two or-
ganisms are: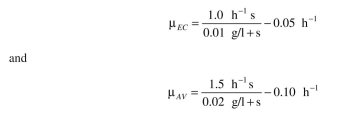 The yield coefficients are:
The yield coefficients are:  The inoculum for the fermenter is 0.03 g dw/l of E. coli
The inoculum for the fermenter is 0.03 g dw/l of E. coli 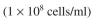 and 0.15 g dw/l of
and 0.15 g dw/l of
A. vinelandii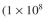 cells/ml).
cells/ml).
What will be the ratio of A. vinelandii to E. coli at the time when all of the glucose is
consumed?
Example 16.1.
Competition of two species for the same growth-rate-limiting substrate is common. Deter- mine when the two organisms may stably coexist if both A and B follow Monod kinetics.
limiting nutrient for a mixed population of two bacteria (a strain of E. coli and Azotobacter
vinelandii). A. vinelandii is five times larger than E. coli. The replication rates for the two or-
ganisms are:
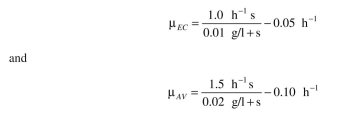 The yield coefficients are:
The yield coefficients are:  The inoculum for the fermenter is 0.03 g dw/l of E. coli
The inoculum for the fermenter is 0.03 g dw/l of E. coli 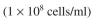 and 0.15 g dw/l of
and 0.15 g dw/l ofA. vinelandii
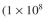 cells/ml).
cells/ml).What will be the ratio of A. vinelandii to E. coli at the time when all of the glucose is
consumed?
Example 16.1.
Competition of two species for the same growth-rate-limiting substrate is common. Deter- mine when the two organisms may stably coexist if both A and B follow Monod kinetics.

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
9
Consider Example 16.1, where we demonstrated that two bacteria competing for a single nu-
trient in a chemostat (well-mixed) could not coexist. Consider the situation where B can ad-
here to a surface but A cannot. Redo the balance equations, where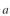 is the surface area
is the surface area
available per unit reactor volume and the rate of attachment is first order in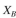 with a rate
with a rate
constant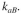 The sites available for attachment will be
The sites available for attachment will be 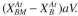 The attached cells can
The attached cells can
detach with a first-order dependence on the attached cell concentration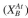 with a rate con-
with a rate con-
stant of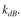 Attached cells grow with the same kinetics as suspended cells.
Attached cells grow with the same kinetics as suspended cells.
a. Without mathematical proofs, do you think coexistence may be possible? Why or
why not?
b. Consider the specific case below and solve the appropriate balance equations for
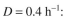
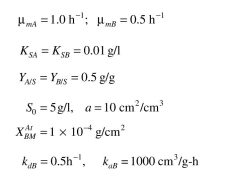
trient in a chemostat (well-mixed) could not coexist. Consider the situation where B can ad-
here to a surface but A cannot. Redo the balance equations, where
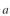 is the surface area
is the surface areaavailable per unit reactor volume and the rate of attachment is first order in
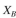 with a rate
with a rateconstant
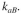 The sites available for attachment will be
The sites available for attachment will be 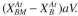 The attached cells can
The attached cells candetach with a first-order dependence on the attached cell concentration
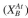 with a rate con-
with a rate con-stant of
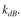 Attached cells grow with the same kinetics as suspended cells.
Attached cells grow with the same kinetics as suspended cells. a. Without mathematical proofs, do you think coexistence may be possible? Why or
why not?
b. Consider the specific case below and solve the appropriate balance equations for
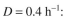

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck


