Deck 14: Utilizing Geneticallyengineered Organisms
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/11
Play
Full screen (f)
Deck 14: Utilizing Geneticallyengineered Organisms
1
Assume that all plasmid-containing cells have eight plasmids; that an antibiotic is present in
the medium, and the plasmid-containing cells are totally resistant; and that a newly born,
plasmid-free cell has sufficient enzyme to protect a cell and its progeny for three generations.
Estimate the fraction of plasmid-containing cells in the population in a batch reactor starting
with only plasmid-containing cells after five generations.
the medium, and the plasmid-containing cells are totally resistant; and that a newly born,
plasmid-free cell has sufficient enzyme to protect a cell and its progeny for three generations.
Estimate the fraction of plasmid-containing cells in the population in a batch reactor starting
with only plasmid-containing cells after five generations.
According to the given data:
As a basis, for the calculation assuming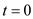 that
that 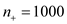 and
and 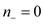
Further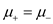 for
for  where i = 1, 2, or 3;
where i = 1, 2, or 3;  = dead cell
= dead cell  0.00781 plasmid-free cells / division
0.00781 plasmid-free cells / division 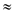 0.008
0.008 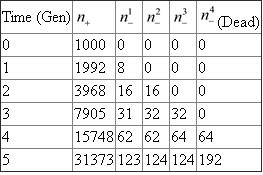 Total plasmid-free cells = 563 or 1.76%
Total plasmid-free cells = 563 or 1.76%
Total Viable plasmid-free cells = 371 or 1.16%
Thus, the total plasmid-free cells are found to be 1.70% and viable plasmid-free cell is found to be 1.16%
As a basis, for the calculation assuming
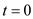 that
that 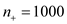 and
and 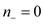
Further
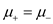 for
for  where i = 1, 2, or 3;
where i = 1, 2, or 3;  = dead cell
= dead cell  0.00781 plasmid-free cells / division
0.00781 plasmid-free cells / division 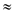 0.008
0.008  Total plasmid-free cells = 563 or 1.76%
Total plasmid-free cells = 563 or 1.76%Total Viable plasmid-free cells = 371 or 1.16%
Thus, the total plasmid-free cells are found to be 1.70% and viable plasmid-free cell is found to be 1.16%
2
Consider an industrial-scale batch fermentation. A 10,000 l fermenter with 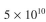 cells/ml
cells/ml
is the desired scale-up operation. Inoculum for the large tank is brought through a series of
seed tanks and flasks, beginning with a single pure colony growing on an agar slant. Assume
that a colony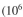 plasmid-containing cells) is picked and placed in a test tube with 1 ml of
plasmid-containing cells) is picked and placed in a test tube with 1 ml of
medium. Calculate how many generations will be required to achieve the required cell den-
sity in the 10,000 l fermenter. What fraction of the total population will be plasmid-free cells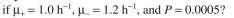
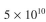 cells/ml
cells/mlis the desired scale-up operation. Inoculum for the large tank is brought through a series of
seed tanks and flasks, beginning with a single pure colony growing on an agar slant. Assume
that a colony
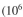 plasmid-containing cells) is picked and placed in a test tube with 1 ml of
plasmid-containing cells) is picked and placed in a test tube with 1 ml ofmedium. Calculate how many generations will be required to achieve the required cell den-
sity in the 10,000 l fermenter. What fraction of the total population will be plasmid-free cells
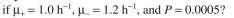
According to the given data:
In industrial-scale batch fementtion 10,000 l fermenter has = 5 × 10 10 cells/ml.
A colony is picked and placed in test tube of 1 ml = 10 6 plasmid-containing cells.
The number of generations required for a cell density = 10,000 l.
To calculate the total number of cells: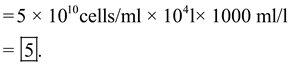 Final cell =
Final cell = 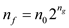
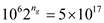 Therefore, generations
Therefore, generations  = 39
= 39
According to the given data, using equation 14.51 to calculate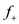 the following calculation is evolved:
the following calculation is evolved: 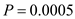 µ + = 1.0 h -1
µ + = 1.0 h -1
µ - = 1.2 h -1
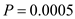
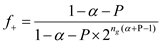

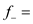
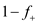 = 0.36.
= 0.36.
Thus, the fraction of total population in plasmid-free cells is 0.36.
In industrial-scale batch fementtion 10,000 l fermenter has = 5 × 10 10 cells/ml.
A colony is picked and placed in test tube of 1 ml = 10 6 plasmid-containing cells.
The number of generations required for a cell density = 10,000 l.
To calculate the total number of cells:
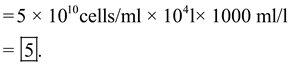 Final cell =
Final cell = 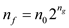
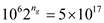 Therefore, generations
Therefore, generations  = 39
= 39According to the given data, using equation 14.51 to calculate
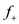 the following calculation is evolved:
the following calculation is evolved: 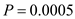 µ + = 1.0 h -1
µ + = 1.0 h -1 µ - = 1.2 h -1

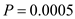
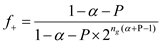

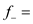
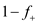 = 0.36.
= 0.36.Thus, the fraction of total population in plasmid-free cells is 0.36.
3
Assume that you have been assigned to a team to produce human epidermal growth factor
(hEGF). A small peptide, hEGF speeds wound healing and may be useful in treating ulcers. A
market size of 50 to 500 kg/yr has been estimated. Posttranslational processing is not essen-
tial to the value of the product. It is a secreted product in the natural host cell. Discuss what
recommendations you would make to the molecular-biology team leader for the choice of
host cell and the design of a reactor. Make your recommendations from the perspective of
what is desirable to make an effective process. You should point out any potential prob-
lems with the solution you have proposed, as well as defend why your approach should be
advantageous.
(hEGF). A small peptide, hEGF speeds wound healing and may be useful in treating ulcers. A
market size of 50 to 500 kg/yr has been estimated. Posttranslational processing is not essen-
tial to the value of the product. It is a secreted product in the natural host cell. Discuss what
recommendations you would make to the molecular-biology team leader for the choice of
host cell and the design of a reactor. Make your recommendations from the perspective of
what is desirable to make an effective process. You should point out any potential prob-
lems with the solution you have proposed, as well as defend why your approach should be
advantageous.
Human epidermal growth factor (hEGF) is assigned to a tem. The growth factor increase the healing and are useful in treating ulcers.
Several choices could be well defined. Since, post-translational processing is not required, efficiency and productivity has become critical. If the protein can be resolublized from inclusion bodies, then E.coli will likely be the best choice, if intracellular degradation is not a problem.
An E.coli system modified, for excretion would be advantageous. Yeast or fungal systems would also be possible alternatives particularly, for internal medical use.
Several choices could be well defined. Since, post-translational processing is not required, efficiency and productivity has become critical. If the protein can be resolublized from inclusion bodies, then E.coli will likely be the best choice, if intracellular degradation is not a problem.
An E.coli system modified, for excretion would be advantageous. Yeast or fungal systems would also be possible alternatives particularly, for internal medical use.
4
Develop a model to describe the stability of a chemostat culture for a plasmid-containing cul-
ture. For some cultures, plasmids make a protein product (e.g., colicin in E. coli) that kills
plasmid-free cells but does not act on plasmid-containing cells. Assume that the rate of
killing by colicin is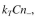 where
where 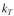 is the rate constant for the killing and C is the colicin
is the rate constant for the killing and C is the colicin
concentration. Assume that the colicin production is first order with respect to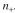
ture. For some cultures, plasmids make a protein product (e.g., colicin in E. coli) that kills
plasmid-free cells but does not act on plasmid-containing cells. Assume that the rate of
killing by colicin is
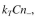 where
where 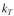 is the rate constant for the killing and C is the colicin
is the rate constant for the killing and C is the colicinconcentration. Assume that the colicin production is first order with respect to
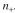

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the following data for E. coli B/r-pDW17 grown in a minimal medium supple-
mented with amino acids. Estimate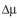 and R. Compare the stability of this system to one
and R. Compare the stability of this system to one
with a glucose-minimal medium (Example 14.2).
Example 14.2.
The data in Table 14.4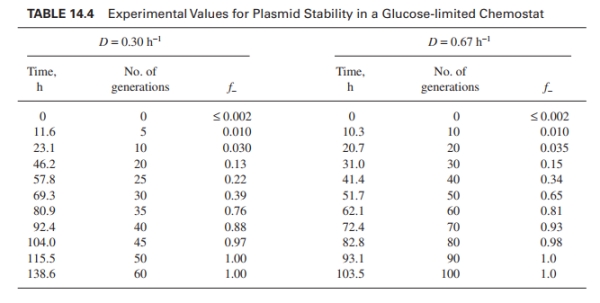 were obtained for E. coli B/r-pDW17 at two different dilution rates in
were obtained for E. coli B/r-pDW17 at two different dilution rates in
glucose-limited chemostats. The average plasmid copy number for pDW17 is about 40 to 50
copies per cell. About 12of the total protein synthesized is due to the plasmid. The proteins
are retained intracellularly in soluble form. Use these data to estimate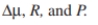
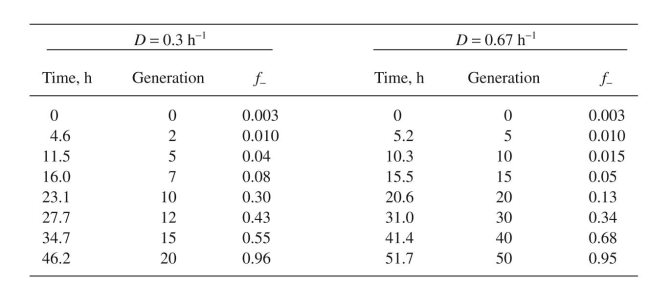
mented with amino acids. Estimate
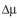 and R. Compare the stability of this system to one
and R. Compare the stability of this system to onewith a glucose-minimal medium (Example 14.2).
Example 14.2.
The data in Table 14.4
 were obtained for E. coli B/r-pDW17 at two different dilution rates in
were obtained for E. coli B/r-pDW17 at two different dilution rates inglucose-limited chemostats. The average plasmid copy number for pDW17 is about 40 to 50
copies per cell. About 12of the total protein synthesized is due to the plasmid. The proteins
are retained intracellularly in soluble form. Use these data to estimate
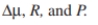


Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
6
It has been claimed that gel immobilization stabilizes a plasmid-containing population. A fac-
tor suggested to be responsible for the stabilization is compartmentalization of the population
into very small pockets. For example, the pocket may start with an individual cell and grow
to a level of 200 cells per cavity. Develop a mathematical formula to compare the number of
plasmid-free cells in a gel to that in a large, well-mixed tank.
tor suggested to be responsible for the stabilization is compartmentalization of the population
into very small pockets. For example, the pocket may start with an individual cell and grow
to a level of 200 cells per cavity. Develop a mathematical formula to compare the number of
plasmid-free cells in a gel to that in a large, well-mixed tank.

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
7
You must design an operating strategy to allow an E. coli fermentation to achieve a high cell
density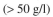 in a fed-batch system. You have access to an off-gas analyzer that will mea-
in a fed-batch system. You have access to an off-gas analyzer that will mea-
sure the pCO₂ in the exit gas. The glucose concentration must be less than 100 mg/l to avoid
the formation of acetate and other inhibitory products. Develop an approach to control the
glucose feed rate so as to maintain the glucose level at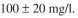 what equations would
what equations would
you use and what assumptions would you make?
density
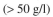 in a fed-batch system. You have access to an off-gas analyzer that will mea-
in a fed-batch system. You have access to an off-gas analyzer that will mea-sure the pCO₂ in the exit gas. The glucose concentration must be less than 100 mg/l to avoid
the formation of acetate and other inhibitory products. Develop an approach to control the
glucose feed rate so as to maintain the glucose level at
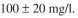 what equations would
what equations wouldyou use and what assumptions would you make?

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
8
Develop a simple model for a population in which plasmids are present at division with copy
numbers 2, 4, 6, 8, or 10. The model should be developed in analogy to eqs. 14.7 through 14.9. You can assume that dividing cells either segregate plasmids perfectly or generate a
plasmid-free cell.
numbers 2, 4, 6, 8, or 10. The model should be developed in analogy to eqs. 14.7 through 14.9. You can assume that dividing cells either segregate plasmids perfectly or generate a
plasmid-free cell.

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
9
Assume you have an inoculum with 95% plasmid-containing cells and 5% plasmid-free cells
in a 2 l reactor with a total cell population of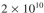 cells/ml. You use this inoculum for a
cells/ml. You use this inoculum for a
1000 l reactor and achieve a final population of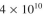 cells/ml. Assuming
cells/ml. Assuming 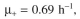
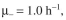 and P = 0.0002, predict the fraction of plasmid-containing cells.
and P = 0.0002, predict the fraction of plasmid-containing cells.
in a 2 l reactor with a total cell population of
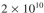 cells/ml. You use this inoculum for a
cells/ml. You use this inoculum for a1000 l reactor and achieve a final population of
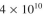 cells/ml. Assuming
cells/ml. Assuming 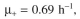
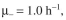 and P = 0.0002, predict the fraction of plasmid-containing cells.
and P = 0.0002, predict the fraction of plasmid-containing cells.
Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
10
Assume you scale up from 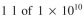 cells/ml of 100% plasmid-containing cells to
cells/ml of 100% plasmid-containing cells to 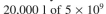 cells/ml, at which point overproduction of the target protein is induced.
cells/ml, at which point overproduction of the target protein is induced.  You harvest six hours after induction. The value of P is 0.0005. Before induction
You harvest six hours after induction. The value of P is 0.0005. Before induction 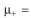
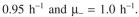 After induction
After induction 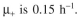 What is the fraction of plasmid-
What is the fraction of plasmid-
containing cells at induction? What is the fraction of plasmid-containing cells at harvest?
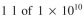 cells/ml of 100% plasmid-containing cells to
cells/ml of 100% plasmid-containing cells to 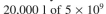 cells/ml, at which point overproduction of the target protein is induced.
cells/ml, at which point overproduction of the target protein is induced.  You harvest six hours after induction. The value of P is 0.0005. Before induction
You harvest six hours after induction. The value of P is 0.0005. Before induction 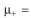
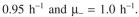 After induction
After induction 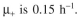 What is the fraction of plasmid-
What is the fraction of plasmid-containing cells at induction? What is the fraction of plasmid-containing cells at harvest?

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
11
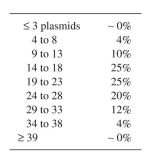
Given the following information, calculate the probability of forming a plasmid-free cell due
to random segregation for a cell with 50 plasmid monomer equivalents:
a. 40% of the total plasmid DNA is in dimers and 16% in tetramers.
b. The distribution of copy numbers per cell is as follows, assuming monomers only:
to random segregation for a cell with 50 plasmid monomer equivalents:
a. 40% of the total plasmid DNA is in dimers and 16% in tetramers.
b. The distribution of copy numbers per cell is as follows, assuming monomers only:


Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck


