Deck 21: Structure and Bonding in Solids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/33
Play
Full screen (f)
Deck 21: Structure and Bonding in Solids
1
Which of the following has 3 non-identical 2-fold rotation axes?
A) A cereal box
B) A five pointed star
C) A cube
D) An isosceles triangle
E) An equilateral triangle
A) A cereal box
B) A five pointed star
C) A cube
D) An isosceles triangle
E) An equilateral triangle
A
2
Which of the following has the most mirror planes?
A) A cereal box
B) A five pointed star
C) A cube
D) An isosceles triangle
E) An equilateral triangle
A) A cereal box
B) A five pointed star
C) A cube
D) An isosceles triangle
E) An equilateral triangle
C
3
Which of the following has exactly one two-fold rotation axis?
A) A cereal box
B) A five pointed star
C) A cube
D) An isosceles triangle
E) An equilateral triangle
A) A cereal box
B) A five pointed star
C) A cube
D) An isosceles triangle
E) An equilateral triangle
D
4
Which of the following have the same number of rotation axes and mirror planes?
A) Cereal box and a cube
B) A five pointed star and an isosceles triangle
C) An isosceles triangle and a five pointed star
D) A pentagon and an isosceles triangle
E) A pentagon and a five pointed star
A) Cereal box and a cube
B) A five pointed star and an isosceles triangle
C) An isosceles triangle and a five pointed star
D) A pentagon and an isosceles triangle
E) A pentagon and a five pointed star

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
5
Of the seven crystal systems, which has the lowest symmetry requirements?
A) hexagonal
B) cubic
C) triclinic
D) tetragonal
E) orthorhombic
A) hexagonal
B) cubic
C) triclinic
D) tetragonal
E) orthorhombic

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
6
The tetragonal crystal system minimally requires one four-fold rotation axis. Which of the following conditions on unit-cell edges and angles would satisfy this?
A) a=b=c, a=b=g=90°
B) a=b¹c, a=b=g=90°
C) a=b=c, a=b=g¹90°
D) a and b
E) a and c
A) a=b=c, a=b=g=90°
B) a=b¹c, a=b=g=90°
C) a=b=c, a=b=g¹90°
D) a and b
E) a and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
7
Assuming that atoms are solid spheres, what percentage of space is consumed by the atoms in the simple cubic primitive cell?
A) 48%
B) 52%
C) 68%
D) 26%
E) 32%
A) 48%
B) 52%
C) 68%
D) 26%
E) 32%

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
8
Assuming that atoms are solid spheres, what percentage of space is consumed by the atoms in the body-centered cubic primitive cell?
A) 48%
B) 52%
C) 68%
D) 26%
E) 74%
A) 48%
B) 52%
C) 68%
D) 26%
E) 74%

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
9
Assuming that atoms are solid spheres, what amount of free space is found in the face-centered cubic primitive cell?
A) 48%
B) 52%
C) 68%
D) 26%
E) 32%
A) 48%
B) 52%
C) 68%
D) 26%
E) 32%

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is not an acceptable location for an atom in a side-centered primitive cell?
A) (1,1,0)
B) (1, ,0)
,0)
C)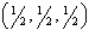
D) all of the above
E) none of the above
A) (1,1,0)
B) (1,
 ,0)
,0)C)
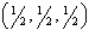
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
11
Exhibit 21-1
The following question(s) pertain to Gallium which has a simple cubic unit cell. It has a molar mass of 69.72 g·mol-1 and a density of 5.91 g·cm-3.
Refer to Exhibit 21-1. How many atoms are contained within a single Gallium unit cell?
A) 1
B) 2
C) 3
D) 4
E) >4
The following question(s) pertain to Gallium which has a simple cubic unit cell. It has a molar mass of 69.72 g·mol-1 and a density of 5.91 g·cm-3.
Refer to Exhibit 21-1. How many atoms are contained within a single Gallium unit cell?
A) 1
B) 2
C) 3
D) 4
E) >4

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
12
Exhibit 21-1
The following question(s) pertain to Gallium which has a simple cubic unit cell. It has a molar mass of 69.72 g·mol-1 and a density of 5.91 g·cm-3.
Refer to Exhibit 21-1. What is the diameter of a single Gallium atom?
A) 2.70 Å
B) 1.35 Å
C) 1.63 Å
D) 2.44 Å
The following question(s) pertain to Gallium which has a simple cubic unit cell. It has a molar mass of 69.72 g·mol-1 and a density of 5.91 g·cm-3.
Refer to Exhibit 21-1. What is the diameter of a single Gallium atom?
A) 2.70 Å
B) 1.35 Å
C) 1.63 Å
D) 2.44 Å

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
13
Exhibit 21-2
The following question(s) pertain to Palladium which has a face-centered cubic unit cell. It has a molar mass of 106.4 g·mol-1 and a density of 7.14 g·cm-3.
Refer to Exhibit 21-2. How many atoms are contained within a single palladium unit cell?
A) 1
B) 2
C) 3
D) 4
E) >4
The following question(s) pertain to Palladium which has a face-centered cubic unit cell. It has a molar mass of 106.4 g·mol-1 and a density of 7.14 g·cm-3.
Refer to Exhibit 21-2. How many atoms are contained within a single palladium unit cell?
A) 1
B) 2
C) 3
D) 4
E) >4

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
14
Exhibit 21-2
The following question(s) pertain to Palladium which has a face-centered cubic unit cell. It has a molar mass of 106.4 g·mol-1 and a density of 7.14 g·cm-3.
Refer to Exhibit 21-2. What is the radius of a single palladium atom?
A) 2.70 Å
B) 1.22 Å
C) 1.64 Å
D) 2.44 Å
The following question(s) pertain to Palladium which has a face-centered cubic unit cell. It has a molar mass of 106.4 g·mol-1 and a density of 7.14 g·cm-3.
Refer to Exhibit 21-2. What is the radius of a single palladium atom?
A) 2.70 Å
B) 1.22 Å
C) 1.64 Å
D) 2.44 Å

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
15
Exhibit 21-3
The following question(s) pertain to Iron which has a body-centered cubic unit cell. It has a molar mass of 55.847 g·mol-1 and a density of 7.86 g·cm-3.
Refer to Exhibit 21-3. How many atoms are contained within a single iron unit cell?
A) 1
B) 2
C) 3
D) 4
E) >4
The following question(s) pertain to Iron which has a body-centered cubic unit cell. It has a molar mass of 55.847 g·mol-1 and a density of 7.86 g·cm-3.
Refer to Exhibit 21-3. How many atoms are contained within a single iron unit cell?
A) 1
B) 2
C) 3
D) 4
E) >4

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
16
Exhibit 21-3
The following question(s) pertain to Iron which has a body-centered cubic unit cell. It has a molar mass of 55.847 g·mol-1 and a density of 7.86 g·cm-3.
Refer to Exhibit 21-3. What is the radius of a single iron atom?
A) 2.55 Å
B) 1.24 Å
C) 1.94 Å
D) 1.05 Å
The following question(s) pertain to Iron which has a body-centered cubic unit cell. It has a molar mass of 55.847 g·mol-1 and a density of 7.86 g·cm-3.
Refer to Exhibit 21-3. What is the radius of a single iron atom?
A) 2.55 Å
B) 1.24 Å
C) 1.94 Å
D) 1.05 Å

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
17
An element crystallizes in a body-centered cubic lattice, with a = 4.109 Å. If the element has a density of 9.95 g·cm-3, what is the molar mass of the element?
A) 104 g·mol-1
B) 208 g·mol-1
C) 416 g·mol-1
D) 831 g·mol-1
A) 104 g·mol-1
B) 208 g·mol-1
C) 416 g·mol-1
D) 831 g·mol-1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
18
In crystalline sodium the distance between adjacent layers in a certain set of equally spaced planes of atoms is 4.28 Å. What is the maximum order n for observation of Bragg diffraction using X-rays with a wavelength of 1.936 Å?
A) 1
B) 2
C) 3
D) 4
E) >4
A) 1
B) 2
C) 3
D) 4
E) >4

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
19
When two metal atoms are brought close together
A) an electron is transferred from one atom to the next resulting in a coulombic attraction.
B) all of the electrons are shared between the two atoms.
C) Groups of closely spaced molecular orbitals are formed.
D) b and c
E) none of the above
A) an electron is transferred from one atom to the next resulting in a coulombic attraction.
B) all of the electrons are shared between the two atoms.
C) Groups of closely spaced molecular orbitals are formed.
D) b and c
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
20
Covalent crystals are
A) soft and malleable.
B) hard and brittle.
C) have low melting points.
D) a and c
E) b and c
A) soft and malleable.
B) hard and brittle.
C) have low melting points.
D) a and c
E) b and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
21
Molecular crystals are
A) soft and malleable
B) hard and brittle
C) have low melting points
D) a and c
E) b and c
A) soft and malleable
B) hard and brittle
C) have low melting points
D) a and c
E) b and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
22
Molecular crystals are held in lattice sites by
A) covalent bonds.
B) ionic bonds.
C) intermolecular forces.
D) large molecular orbitals that span many lattice sites.
E) a and c
A) covalent bonds.
B) ionic bonds.
C) intermolecular forces.
D) large molecular orbitals that span many lattice sites.
E) a and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is a metalloid?
A) Al
B) Ge
C) As
D) All of the above
E) None of the above
A) Al
B) Ge
C) As
D) All of the above
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
24
The hybridization of carbon in graphite is ______ while in diamond it is _______.
A) sp2, sp2
B) sp2, sp3
C) sp2, sp3d
D) sp3, sp3d2
E) None of the above
A) sp2, sp2
B) sp2, sp3
C) sp2, sp3d
D) sp3, sp3d2
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
25
Schottky defects are a result of
A) vacancies in the crystal lattice
B) atoms inserted at interstitial sites
C) electron transfer
D) p-bonding interactions at the surface
E) a and b
A) vacancies in the crystal lattice
B) atoms inserted at interstitial sites
C) electron transfer
D) p-bonding interactions at the surface
E) a and b

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
26
Frenkel defects are a result of
A) vacancies in the crystal lattice
B) atoms inserted at interstitial sites
C) electron transfer
D) p-bonding interactions at the surface
E) a and b
A) vacancies in the crystal lattice
B) atoms inserted at interstitial sites
C) electron transfer
D) p-bonding interactions at the surface
E) a and b

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
27
Crystalline substances are _______. Amorphous substances are _______.
A) Isotropic, isotropic
B) Anisotropic, anisotropic.
C) Isotropic, anisotropic.
D) Anisotropic, isotropic.
A) Isotropic, isotropic
B) Anisotropic, anisotropic.
C) Isotropic, anisotropic.
D) Anisotropic, isotropic.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
28
For a face-centered cubic lattice, each lattice point located in the face of the unit cell is shared equally with _____ other cells.
A) 1
B) 2
C) 4
D) 8
A) 1
B) 2
C) 4
D) 8

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
29
For a face-centered cubic lattice, each lattice point located in the corner of the unit cell is shared equally with _____ other cells.
A) 1
B) 2
C) 4
D) 8
A) 1
B) 2
C) 4
D) 8

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
30
For a body-centered unit cell, each corner contributes _____ lattice points to the unit cell.
A) 1/8
B) 1/4
C) 1/2
D) 1
A) 1/8
B) 1/4
C) 1/2
D) 1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
31
Nonstoichiometric compounds can contain
A) different atomic elements that are not in whole number proportions to each other.
B) identical atoms with different oxidation states.
C) species that, upon reaction, always produce less than the theoretical yield.
D) A and B only
E) none of the above
A) different atomic elements that are not in whole number proportions to each other.
B) identical atoms with different oxidation states.
C) species that, upon reaction, always produce less than the theoretical yield.
D) A and B only
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
32
Alloy steels can be described as
A) substitutional
B) amorphous
C) interstitial
D) A and B
E) A and C
A) substitutional
B) amorphous
C) interstitial
D) A and B
E) A and C

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
33
Amorphous solids may have the following:
A) ionic bonding
B) metallic bonding
C) covalent bonding
D) molecular bonding
E) all of the above
A) ionic bonding
B) metallic bonding
C) covalent bonding
D) molecular bonding
E) all of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck


