Deck 20: Molecular Spectroscopy and Photochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/33
Play
Full screen (f)
Deck 20: Molecular Spectroscopy and Photochemistry
1
A heteronuclear diatomic molecule has m1 < m2. The center of gravity for the molecule is therefore closer to m1.
False
2
A diatomic molecule will always rotate about its
A) moment of inertia
B) center of gravity
C) all of the above
D) none of the above
A) moment of inertia
B) center of gravity
C) all of the above
D) none of the above
B
3
How many moments of inertia can a polyatomic molecule have?
A) 1
B) 2
C) 3
D) Up to 3N, where N is the number of atoms in the molecule
A) 1
B) 2
C) 3
D) Up to 3N, where N is the number of atoms in the molecule
C
4
Which of the following will affect the rotational constant, B:
A) Masses of atoms in the molecule
B) Bond lengths
C) Moment of inertia
D) All of the above
E) None of the above
A) Masses of atoms in the molecule
B) Bond lengths
C) Moment of inertia
D) All of the above
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
5
Which type of molecular spectroscopy is used to determine force constants in molecules?
A) Rotational
B) vibrational
C) electronic
D) a and b
E) all of the above
A) Rotational
B) vibrational
C) electronic
D) a and b
E) all of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
6
Of the following types of radiation, which has the longest wavelength?
A) radio waves
B) microwaves
C) X-ray
D) visible
E) infrared
A) radio waves
B) microwaves
C) X-ray
D) visible
E) infrared

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
7
Of the following types of radiation, which has the highest frequency?
A) radio waves
B) microwaves
C) X-ray
D) visible
E) infrared
A) radio waves
B) microwaves
C) X-ray
D) visible
E) infrared

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
8
Of the following types of radiation, which has the highest energy?
A) radio waves
B) microwaves
C) X-ray
D) visible
E) infrared
A) radio waves
B) microwaves
C) X-ray
D) visible
E) infrared

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
9
The zero point energy in vibrational spectroscopy is equal to
A) 2B
B) 1/2 hv
C) hv
D) hc
E) none of the above
A) 2B
B) 1/2 hv
C) hv
D) hc
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
10
In the parabolic approximation used to solve the vibrational Schrödinger equation for diatomic molecules, the spacing between energy levels is equal to
A) 2B
B) 1/2 hv
C) hv
D) hc
E) none of the above
A) 2B
B) 1/2 hv
C) hv
D) hc
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
11
At high vibrational levels, the spacing between the vibrational energy levels in the Morse potential
A) increases
B) decreases
C) asymptotically approaches zero
D) b and c
E) stays constant
A) increases
B) decreases
C) asymptotically approaches zero
D) b and c
E) stays constant

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
12
What is the reduced mass of the NO molecule
A) 16 u
B) 14 u
C) 7.5 u
D) 0.13 u
A) 16 u
B) 14 u
C) 7.5 u
D) 0.13 u

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
13
Which type of radiation is used in vibrational spectroscopy?
A) radio waves
B) microwaves
C) X-ray
D) visible
E) infrared
A) radio waves
B) microwaves
C) X-ray
D) visible
E) infrared

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
14
Molecules in excited electronic states can return to the ground electronic state by emitting a photon through the processes of fluorescence and phosphorescence. Which of these processes is faster?
A) fluorescence
B) phosphorescence
C) they occur at the same rate
D) more information is required to answer this question
A) fluorescence
B) phosphorescence
C) they occur at the same rate
D) more information is required to answer this question

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
15
Lasers operate through the process of
A) fluorescence
B) spontaneous emission
C) stimulated emission
D) a and b
E) more information is required to answer this question
A) fluorescence
B) spontaneous emission
C) stimulated emission
D) a and b
E) more information is required to answer this question

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following will have the maximum ultraviolet absorbance at the longest wavelength?
A)
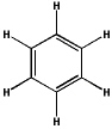
B)
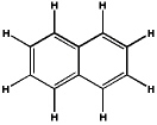
C)
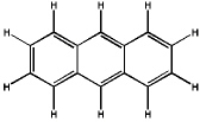
D)
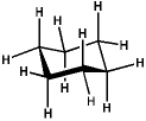
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
17
Temperature increases with increasing altitude in the region of the atmosphere known as the
A) troposphere
B) stratosphere
C) mesosphere
D) all of the above
E) none of the above
A) troposphere
B) stratosphere
C) mesosphere
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
18
The destruction of ozone (O3) typically takes place in the
A) troposphere
B) stratosphere
C) mesosphere
D) all of the above
E) none of the above
A) troposphere
B) stratosphere
C) mesosphere
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following molecules significantly absorb infrared radiation and therefore contribute to the "greenhouse effect"?
A) N2
B) CO2
C) CH4
D) a and b
E) b and c
A) N2
B) CO2
C) CH4
D) a and b
E) b and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
20
The Beer-Lambert law states that concentration is directly proportional to
A) absorbance.
B) transmittance.
C) molar absorptivity.
D) path length.
E) a and b
A) absorbance.
B) transmittance.
C) molar absorptivity.
D) path length.
E) a and b

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
21
The moment of inertia in a diatomic molecule is directly proportional to the
A) bond length
B) square of the bond length
C) square of the reduced mass of the molecule
D) a and c
E) b and c
A) bond length
B) square of the bond length
C) square of the reduced mass of the molecule
D) a and c
E) b and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
22
In a rigid diatomic molecule, the rotational lines are uniformly separated in frequency by a value of
A) B
B) 2hv
C) 2h/(8p2l)
D) 2B
E) a and c
F) c and d
A) B
B) 2hv
C) 2h/(8p2l)
D) 2B
E) a and c
F) c and d

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
23
Bond force constants determine
A) bond strength
B) bond stiffness
C) molecular polarizability
D) moments of inertia
E) a and b
A) bond strength
B) bond stiffness
C) molecular polarizability
D) moments of inertia
E) a and b

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
24
The area under the peaks in an NMR spectrum are proportional to
A) The number of hydrogens atoms in a particular chemical environment
B) The number of hydrogen atoms in the molecule experiencing "spin flips"
C) The number of hydrogen atoms with electrons in excited electronic states
D) a and c
E) none of the above
A) The number of hydrogens atoms in a particular chemical environment
B) The number of hydrogen atoms in the molecule experiencing "spin flips"
C) The number of hydrogen atoms with electrons in excited electronic states
D) a and c
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
25
Which type of radiation is used in nuclear magnetic resonance spectroscopy?
A) radio waves
B) microwaves
C) X-ray
D) visible
E) infrared
A) radio waves
B) microwaves
C) X-ray
D) visible
E) infrared

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
26
Spectroscopic experiments in the visible region of the spectrum involve excitation of
A) core electrons
B) valence electrons
C) nuclear spins
D) vibrational levels
E) none of the above
A) core electrons
B) valence electrons
C) nuclear spins
D) vibrational levels
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
27
Spectroscopic experiments in the X-Ray region of the spectrum involve excitation of
A) core electrons
B) valence electrons
C) nuclear spins
D) vibrational levels
E) none of the above
A) core electrons
B) valence electrons
C) nuclear spins
D) vibrational levels
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following statements are true regarding chlorofluorocarbons (CFC's)?
A) They are unreactive in the troposphere.
B) They photodissociate to form chlorine atoms in the stratosphere.
C) They persist for a long time in the stratosphere.
D) All of the above
E) none of the above
A) They are unreactive in the troposphere.
B) They photodissociate to form chlorine atoms in the stratosphere.
C) They persist for a long time in the stratosphere.
D) All of the above
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
29
The lowest layer of the atmosphere is called the
A) thermosphere
B) stratosphere
C) troposphere
D) mesosphere
A) thermosphere
B) stratosphere
C) troposphere
D) mesosphere

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
30
The leaves of green plants appear green because they absorb this color of light.
A) green
B) violet
C) red
D) b and c
E) none of the above
A) green
B) violet
C) red
D) b and c
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
31
Absorption and emission are
A) single electron processes
B) two-electron processes
C) two-photon processes
D) processes that only allow for exactly one combination of angular momentum
E) all of the above
A) single electron processes
B) two-electron processes
C) two-photon processes
D) processes that only allow for exactly one combination of angular momentum
E) all of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
32
Which molecule should have its proton NMR peak shifted most downfield relative to TMS?
A) CH2I2
B) CH2Cl2
C) CHCl3
D) CH2Br2
E) Not enough information to answer the question
A) CH2I2
B) CH2Cl2
C) CHCl3
D) CH2Br2
E) Not enough information to answer the question

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
33
Of the following, which is an extremely powerful oxidizer that participates in many tropospheric chemical processes?
A) carbon monoxide
B) hydroperoxyl radicals
C) hydroxyl radicals
D) peroxyacylnitrates
E) water
A) carbon monoxide
B) hydroperoxyl radicals
C) hydroxyl radicals
D) peroxyacylnitrates
E) water

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck


