Deck 18: Chemical Kinetics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/33
Play
Full screen (f)
Deck 18: Chemical Kinetics
1
The forward reaction AB → A + B is an example of a
A) Bimolecular decomposition
B) Unimolecular decomposition
C) Termolecular reaction
D) None of the above
A) Bimolecular decomposition
B) Unimolecular decomposition
C) Termolecular reaction
D) None of the above
B
2
Which of the following will a catalyst affect?
A) Activation Energy
B) Enthalpy of Reaction
C) Final reaction concentrations
D) all of the above
E) none of the above
A) Activation Energy
B) Enthalpy of Reaction
C) Final reaction concentrations
D) all of the above
E) none of the above
A
3
Which of the following will an inhibitor affect?
A) Activation Energy
B) Rate constant
C) Reaction mechanism
D) All of the above
E) None of the above
A) Activation Energy
B) Rate constant
C) Reaction mechanism
D) All of the above
E) None of the above
D
4
An uncatalyzed reaction has a DH of -44 kJ and the activation energy for the forward reaction is 21 kJ. What is the activation energy for the reverse reaction?
A) 21 kJ
B) 65 kJ
C) 44 kJ
D) 23 kJ
A) 21 kJ
B) 65 kJ
C) 44 kJ
D) 23 kJ

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
5
Termolecular reactions are more likely to occur than
A) Unimolecular decompositions
B) Bimolecular reactions
C) All of the above
D) None of the above
A) Unimolecular decompositions
B) Bimolecular reactions
C) All of the above
D) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
6
For a first order process, the product of the rate constant and the half life is
A) the rate
B) the molecularity
C) 1
D) ln 10
E) none of these
A) the rate
B) the molecularity
C) 1
D) ln 10
E) none of these

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
7
The powers in the rate law are determined by
A) the coefficients of the balanced chemical equation.
B) the physical states of the reactants and products.
C) the principle of detailed balance.
D) experiment.
E) more that one of these are correct
A) the coefficients of the balanced chemical equation.
B) the physical states of the reactants and products.
C) the principle of detailed balance.
D) experiment.
E) more that one of these are correct

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
8
Reaction rates can change with
A) temperature.
B) the addition of a catalyst.
C) reactant concentrations.
D) all of these
E) none of these
A) temperature.
B) the addition of a catalyst.
C) reactant concentrations.
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
9
A certain first order reaction has a half life of 20.5 minutes. What is the value of the rate constant in s-1.
A) 3.38×10-2
B) 5.64×10-4
C) 1.47×10-2
D) 3.38×10-4
A) 3.38×10-2
B) 5.64×10-4
C) 1.47×10-2
D) 3.38×10-4

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
10
Exhibit 18-1
The following problem(s) pertain to the initial rate data given below for the reaction A2 → 2A.
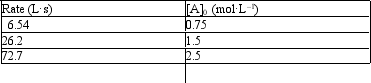 Refer to Exhibit 18-1. What is the order of the reaction?
Refer to Exhibit 18-1. What is the order of the reaction?
A) 0
B) 1
C) 2
D) Not enough data to answer the question
E) None of the above
The following problem(s) pertain to the initial rate data given below for the reaction A2 → 2A.
 Refer to Exhibit 18-1. What is the order of the reaction?
Refer to Exhibit 18-1. What is the order of the reaction?A) 0
B) 1
C) 2
D) Not enough data to answer the question
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
11
Exhibit 18-1
The following problem(s) pertain to the initial rate data given below for the reaction A2 → 2A.
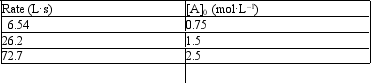 Refer to Exhibit 18-1. What is the value of the rate constant?
Refer to Exhibit 18-1. What is the value of the rate constant?
A) .116
B) 11.6
C) 0.086
D) Not enough data to answer the question
E) None of the above
The following problem(s) pertain to the initial rate data given below for the reaction A2 → 2A.
 Refer to Exhibit 18-1. What is the value of the rate constant?
Refer to Exhibit 18-1. What is the value of the rate constant?A) .116
B) 11.6
C) 0.086
D) Not enough data to answer the question
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
12
Exhibit 18-1
The following problem(s) pertain to the initial rate data given below for the reaction A2 → 2A.
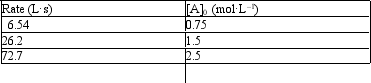 Refer to Exhibit 18-1. What are the units for the rate constant?
Refer to Exhibit 18-1. What are the units for the rate constant?
A) s-1
B) mol·L-1·s-1
C) L·mol-1·s-1
D) L2·mol-2·s-1
E) Not enough data to answer the question
The following problem(s) pertain to the initial rate data given below for the reaction A2 → 2A.
 Refer to Exhibit 18-1. What are the units for the rate constant?
Refer to Exhibit 18-1. What are the units for the rate constant?A) s-1
B) mol·L-1·s-1
C) L·mol-1·s-1
D) L2·mol-2·s-1
E) Not enough data to answer the question

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
13
Exhibit 18-2
The following question(s) pertain to the initial rate data given below for the reaction:
 Refer to Exhibit 18-2. What is the order of the reaction with respect to AB, B, and C?
Refer to Exhibit 18-2. What is the order of the reaction with respect to AB, B, and C?
A) 1,1,1
B) 2,1,1
C) 1,2,1
D) 1,1,2
E) 1,3,1
The following question(s) pertain to the initial rate data given below for the reaction:
 Refer to Exhibit 18-2. What is the order of the reaction with respect to AB, B, and C?
Refer to Exhibit 18-2. What is the order of the reaction with respect to AB, B, and C?A) 1,1,1
B) 2,1,1
C) 1,2,1
D) 1,1,2
E) 1,3,1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
14
Exhibit 18-2
The following question(s) pertain to the initial rate data given below for the reaction:
 Refer to Exhibit 18-2. What is the overall order of the reaction?
Refer to Exhibit 18-2. What is the overall order of the reaction?
A) 1
B) 2
C) 3
D) 4
E) 5
The following question(s) pertain to the initial rate data given below for the reaction:
 Refer to Exhibit 18-2. What is the overall order of the reaction?
Refer to Exhibit 18-2. What is the overall order of the reaction?A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
15
Exhibit 18-2
The following question(s) pertain to the initial rate data given below for the reaction:
 Refer to Exhibit 18-2. What is the value of the rate constant?
Refer to Exhibit 18-2. What is the value of the rate constant?
A) 4
B) 3
C) 3.33
D) 18
E) None of the above
The following question(s) pertain to the initial rate data given below for the reaction:
 Refer to Exhibit 18-2. What is the value of the rate constant?
Refer to Exhibit 18-2. What is the value of the rate constant?A) 4
B) 3
C) 3.33
D) 18
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
16
Exhibit 18-2
The following question(s) pertain to the initial rate data given below for the reaction:
 Refer to Exhibit 18-2. What are the units of the rate constant?
Refer to Exhibit 18-2. What are the units of the rate constant?
A) s-1
B) mol·L-1·s-1
C) L·mol-1·s-1
D) L2·mol-2·s-1
E) L3·mol-3·s-1
The following question(s) pertain to the initial rate data given below for the reaction:
 Refer to Exhibit 18-2. What are the units of the rate constant?
Refer to Exhibit 18-2. What are the units of the rate constant?A) s-1
B) mol·L-1·s-1
C) L·mol-1·s-1
D) L2·mol-2·s-1
E) L3·mol-3·s-1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
17
The rate constant for a first order reaction has the value of 1.25×10-3s-1. If the initial concentration of reactant is 2.5 mol·L-1, what will be the molar concentration of the reactant after 5 minutes?
A) 2.31
B) 1.72
C) 2.49
D) 0.78
E) none of the above
A) 2.31
B) 1.72
C) 2.49
D) 0.78
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
18
The rate constant for a second order reaction has the value of 2.5×10-3 L·mol-1·s-1. If the initial concentration of reactant is 3.5 mol·L-1, what will be the molar concentration of the reactant after 2.5 minutes?
A) 0.97
B) 1.5
C) 3.35
D) 2.33
E) none of the above
A) 0.97
B) 1.5
C) 3.35
D) 2.33
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
19
Chloroethane decomposes at elevated temperatures according to the reaction
C2H5Cl(g) → C2H4(g) + HCl(g)
The reaction obeys first order kinetics. After 340 seconds at 800 K, a measurement shows that the concentration of C2H5Cl decreased from 0.0098 mol·L-1 to 0.0016 mol·L-1. What is the value of the rate constant at 800 K?
A) 7.4×10-4
B) 2.3×10-3
C) 2.0×10-4
D) More data is needed to answer this question
E) none of the above
C2H5Cl(g) → C2H4(g) + HCl(g)
The reaction obeys first order kinetics. After 340 seconds at 800 K, a measurement shows that the concentration of C2H5Cl decreased from 0.0098 mol·L-1 to 0.0016 mol·L-1. What is the value of the rate constant at 800 K?
A) 7.4×10-4
B) 2.3×10-3
C) 2.0×10-4
D) More data is needed to answer this question
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
20
Exhibit 18-3 The following question(s) pertain to the reaction of propene (C3CHCH2) with hydrochloric acid. ![<strong>Exhibit 18-3 The following question(s) pertain to the reaction of propene (C3CHCH2) with hydrochloric acid. A proposed mechanism for this chemical reaction is Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?</strong> A) B) k<sub>3</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH3<sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11ed398b_834a_1151_a786_37e499a4ad5d_TB10703_00.jpg) A proposed mechanism for this chemical reaction is
A proposed mechanism for this chemical reaction is ![<strong>Exhibit 18-3 The following question(s) pertain to the reaction of propene (C3CHCH2) with hydrochloric acid. A proposed mechanism for this chemical reaction is Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?</strong> A) B) k<sub>3</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH3<sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11ed398b_834a_1152_a786_21425642d4a8_TB10703_00.jpg) Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?
Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?
A)
![<strong>Exhibit 18-3 The following question(s) pertain to the reaction of propene (C3CHCH2) with hydrochloric acid. A proposed mechanism for this chemical reaction is Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?</strong> A) B) k<sub>3</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH3<sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11ed398b_834a_1153_a786_79fb7989baba_TB10703_11.jpg)
B) k3[H2Cl2][CH3CHClCH3*]
C) k2[HCl][CH3CHCH2]
D) none of the above
![<strong>Exhibit 18-3 The following question(s) pertain to the reaction of propene (C3CHCH2) with hydrochloric acid. A proposed mechanism for this chemical reaction is Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?</strong> A) B) k<sub>3</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH3<sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11ed398b_834a_1151_a786_37e499a4ad5d_TB10703_00.jpg) A proposed mechanism for this chemical reaction is
A proposed mechanism for this chemical reaction is ![<strong>Exhibit 18-3 The following question(s) pertain to the reaction of propene (C3CHCH2) with hydrochloric acid. A proposed mechanism for this chemical reaction is Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?</strong> A) B) k<sub>3</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH3<sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11ed398b_834a_1152_a786_21425642d4a8_TB10703_00.jpg) Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?
Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?A)
![<strong>Exhibit 18-3 The following question(s) pertain to the reaction of propene (C3CHCH2) with hydrochloric acid. A proposed mechanism for this chemical reaction is Refer to Exhibit 18-3. What is the predicted rate law for this mechanism?</strong> A) B) k<sub>3</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH3<sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11ed398b_834a_1153_a786_79fb7989baba_TB10703_11.jpg)
B) k3[H2Cl2][CH3CHClCH3*]
C) k2[HCl][CH3CHCH2]
D) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
21
Exhibit 18-3 The following question(s) pertain to the reaction of propene (C3CHCH2) with hydrochloric acid. ![<strong>Exhibit 18-3 The following question(s) pertain to the reaction of propene (C3CHCH2) with hydrochloric acid. A proposed mechanism for this chemical reaction is Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step? </strong> A) B) k<sub>2</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH<sub>3</sub><sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11ed398b_834a_3864_a786_0959c28a6c42_TB10703_00.jpg) A proposed mechanism for this chemical reaction is
A proposed mechanism for this chemical reaction is ![<strong>Exhibit 18-3 The following question(s) pertain to the reaction of propene (C3CHCH2) with hydrochloric acid. A proposed mechanism for this chemical reaction is Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step? </strong> A) B) k<sub>2</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH<sub>3</sub><sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11ed398b_834a_3865_a786_51e9a93dbbe4_TB10703_00.jpg) Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step?
Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step?
A)
![<strong>Exhibit 18-3 The following question(s) pertain to the reaction of propene (C3CHCH2) with hydrochloric acid. A proposed mechanism for this chemical reaction is Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step? </strong> A) B) k<sub>2</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH<sub>3</sub><sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11ed398b_834a_3866_a786_91a3cf800bcb_TB10703_11.jpg)
B) k2[H2Cl2][CH3CHClCH3*]
C) k2[HCl][CH3CHCH2]
D) none of the above
![<strong>Exhibit 18-3 The following question(s) pertain to the reaction of propene (C3CHCH2) with hydrochloric acid. A proposed mechanism for this chemical reaction is Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step? </strong> A) B) k<sub>2</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH<sub>3</sub><sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11ed398b_834a_3864_a786_0959c28a6c42_TB10703_00.jpg) A proposed mechanism for this chemical reaction is
A proposed mechanism for this chemical reaction is ![<strong>Exhibit 18-3 The following question(s) pertain to the reaction of propene (C3CHCH2) with hydrochloric acid. A proposed mechanism for this chemical reaction is Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step? </strong> A) B) k<sub>2</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH<sub>3</sub><sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11ed398b_834a_3865_a786_51e9a93dbbe4_TB10703_00.jpg) Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step?
Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step? A)
![<strong>Exhibit 18-3 The following question(s) pertain to the reaction of propene (C3CHCH2) with hydrochloric acid. A proposed mechanism for this chemical reaction is Refer to Exhibit 18-3. What would be the predicted rate law if the second step were the slow step? </strong> A) B) k<sub>2</sub>[H<sub>2</sub>Cl<sub>2</sub>][CH<sub>3</sub>CHClCH<sub>3</sub><sup>*</sup>] C) k<sub>2</sub>[HCl][CH<sub>3</sub>CHCH<sub>2</sub>] D) none of the above](https://storage.examlex.com/TB10703/11ed398b_834a_3866_a786_91a3cf800bcb_TB10703_11.jpg)
B) k2[H2Cl2][CH3CHClCH3*]
C) k2[HCl][CH3CHCH2]
D) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
22
Exhibit 18-3 The following question(s) pertain to the reaction of propene (C3CHCH2) with hydrochloric acid. 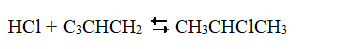 A proposed mechanism for this chemical reaction is
A proposed mechanism for this chemical reaction is  Refer to Exhibit 18-3. If the first step were the slow step, what would be the predicted order of the reaction with respect to propene?
Refer to Exhibit 18-3. If the first step were the slow step, what would be the predicted order of the reaction with respect to propene?
A) 0
B) 1
C) 2
D) Experimental data required to answer this question
E) none of the above
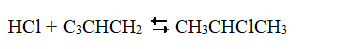 A proposed mechanism for this chemical reaction is
A proposed mechanism for this chemical reaction is  Refer to Exhibit 18-3. If the first step were the slow step, what would be the predicted order of the reaction with respect to propene?
Refer to Exhibit 18-3. If the first step were the slow step, what would be the predicted order of the reaction with respect to propene?A) 0
B) 1
C) 2
D) Experimental data required to answer this question
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
23
Exhibit 18-3 The following question(s) pertain to the reaction of propene (C3CHCH2) with hydrochloric acid. 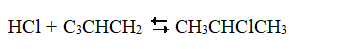 A proposed mechanism for this chemical reaction is
A proposed mechanism for this chemical reaction is  Refer to Exhibit 18-3. Which of the species in the mechanism given above are reaction intermediates?
Refer to Exhibit 18-3. Which of the species in the mechanism given above are reaction intermediates?
A) H2Cl2
B) HCl
C) CH3CHClCH3*
D) a and b
E) none of the above
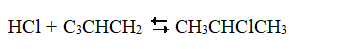 A proposed mechanism for this chemical reaction is
A proposed mechanism for this chemical reaction is  Refer to Exhibit 18-3. Which of the species in the mechanism given above are reaction intermediates?
Refer to Exhibit 18-3. Which of the species in the mechanism given above are reaction intermediates?A) H2Cl2
B) HCl
C) CH3CHClCH3*
D) a and b
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
24
Exhibit 18-4
The following question(s) pertain to the kinetic data given in the table below for the first order reaction:
 Refer to Exhibit 18-4. What is the value of the rate constant in experiment 1?
Refer to Exhibit 18-4. What is the value of the rate constant in experiment 1?
A) 83
B) 0.012
C) 0.12
D) none of the above
The following question(s) pertain to the kinetic data given in the table below for the first order reaction:
 Refer to Exhibit 18-4. What is the value of the rate constant in experiment 1?
Refer to Exhibit 18-4. What is the value of the rate constant in experiment 1?A) 83
B) 0.012
C) 0.12
D) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
25
Exhibit 18-4
The following question(s) pertain to the kinetic data given in the table below for the first order reaction:
 Refer to Exhibit 18-4. What is the value of the activation energy?
Refer to Exhibit 18-4. What is the value of the activation energy?
A) 10.2 kJ
B) 1.15 kJ
C) 14.4 kJ
D) not enough data to answer the question
E) none of the above
The following question(s) pertain to the kinetic data given in the table below for the first order reaction:
 Refer to Exhibit 18-4. What is the value of the activation energy?
Refer to Exhibit 18-4. What is the value of the activation energy?A) 10.2 kJ
B) 1.15 kJ
C) 14.4 kJ
D) not enough data to answer the question
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
26
Exhibit 18-4
The following question(s) pertain to the kinetic data given in the table below for the first order reaction:
 Refer to Exhibit 18-4. What is the value of the pre-exponential term in the Arrhenius equation?
Refer to Exhibit 18-4. What is the value of the pre-exponential term in the Arrhenius equation?
A) 1.2
B) 12
C) 0.012
D) not enough data to answer the question
E) none of the above
The following question(s) pertain to the kinetic data given in the table below for the first order reaction:
 Refer to Exhibit 18-4. What is the value of the pre-exponential term in the Arrhenius equation?
Refer to Exhibit 18-4. What is the value of the pre-exponential term in the Arrhenius equation?A) 1.2
B) 12
C) 0.012
D) not enough data to answer the question
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
27
Exhibit 18-4
The following question(s) pertain to the kinetic data given in the table below for the first order reaction:
 Refer to Exhibit 18-4. What temperature is required to achieve the rate in experiment 3?
Refer to Exhibit 18-4. What temperature is required to achieve the rate in experiment 3?
A) 154°C
B) 212°C
C) 245°C
D) 312°C
E) none of the above
The following question(s) pertain to the kinetic data given in the table below for the first order reaction:
 Refer to Exhibit 18-4. What temperature is required to achieve the rate in experiment 3?
Refer to Exhibit 18-4. What temperature is required to achieve the rate in experiment 3?A) 154°C
B) 212°C
C) 245°C
D) 312°C
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
28
Exhibit 18-5
The following question(s) pertain to the scenario below.
A student performed a kinetic study of the reaction:
2NO(g) + O2(g) → 2NO2(g)
The student prepared the four plots seen below in lab, but forgot to label the axes. A classmate later told him that the reaction was second order in NO and first order in O2. Not wanting to get a bad grade in the class, the student sets out to label the axes on the graphs.![<strong>Exhibit 18-5 The following question(s) pertain to the scenario below. A student performed a kinetic study of the reaction: 2NO(g) + O<sub>2</sub>(g) → 2NO<sub>2</sub>(g) The student prepared the four plots seen below in lab, but forgot to label the axes. A classmate later told him that the reaction was second order in NO and first order in O<sub>2</sub>. Not wanting to get a bad grade in the class, the student sets out to label the axes on the graphs. Refer to Exhibit 18-5. Which graph corresponds to plotting ln[NO] vs. time?</strong> A) top left B) top right C) bottom left D) bottom right E) none of the above](https://storage.examlex.com/TB10703/11ed398b_834b_bf0f_a786_6726e4a2be7e_TB10703_00.jpg) Refer to Exhibit 18-5. Which graph corresponds to plotting ln[NO] vs. time?
Refer to Exhibit 18-5. Which graph corresponds to plotting ln[NO] vs. time?
A) top left
B) top right
C) bottom left
D) bottom right
E) none of the above
The following question(s) pertain to the scenario below.
A student performed a kinetic study of the reaction:
2NO(g) + O2(g) → 2NO2(g)
The student prepared the four plots seen below in lab, but forgot to label the axes. A classmate later told him that the reaction was second order in NO and first order in O2. Not wanting to get a bad grade in the class, the student sets out to label the axes on the graphs.
![<strong>Exhibit 18-5 The following question(s) pertain to the scenario below. A student performed a kinetic study of the reaction: 2NO(g) + O<sub>2</sub>(g) → 2NO<sub>2</sub>(g) The student prepared the four plots seen below in lab, but forgot to label the axes. A classmate later told him that the reaction was second order in NO and first order in O<sub>2</sub>. Not wanting to get a bad grade in the class, the student sets out to label the axes on the graphs. Refer to Exhibit 18-5. Which graph corresponds to plotting ln[NO] vs. time?</strong> A) top left B) top right C) bottom left D) bottom right E) none of the above](https://storage.examlex.com/TB10703/11ed398b_834b_bf0f_a786_6726e4a2be7e_TB10703_00.jpg) Refer to Exhibit 18-5. Which graph corresponds to plotting ln[NO] vs. time?
Refer to Exhibit 18-5. Which graph corresponds to plotting ln[NO] vs. time?A) top left
B) top right
C) bottom left
D) bottom right
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
29
Exhibit 18-5
The following question(s) pertain to the scenario below.
A student performed a kinetic study of the reaction:
2NO(g) + O2(g) → 2NO2(g)
The student prepared the four plots seen below in lab, but forgot to label the axes. A classmate later told him that the reaction was second order in NO and first order in O2. Not wanting to get a bad grade in the class, the student sets out to label the axes on the graphs.![<strong>Exhibit 18-5 The following question(s) pertain to the scenario below. A student performed a kinetic study of the reaction: 2NO(g) + O<sub>2</sub>(g) → 2NO<sub>2</sub>(g) The student prepared the four plots seen below in lab, but forgot to label the axes. A classmate later told him that the reaction was second order in NO and first order in O<sub>2</sub>. Not wanting to get a bad grade in the class, the student sets out to label the axes on the graphs. Refer to Exhibit 18-5. Which graph corresponds to plotting ln[O<sub>2</sub>] vs. time?</strong> A) top left B) top right C) bottom left D) bottom right E) none of the above](https://storage.examlex.com/TB10703/11ed398b_834b_bf10_a786_abd83866d648_TB10703_00.jpg) Refer to Exhibit 18-5. Which graph corresponds to plotting ln[O2] vs. time?
Refer to Exhibit 18-5. Which graph corresponds to plotting ln[O2] vs. time?
A) top left
B) top right
C) bottom left
D) bottom right
E) none of the above
The following question(s) pertain to the scenario below.
A student performed a kinetic study of the reaction:
2NO(g) + O2(g) → 2NO2(g)
The student prepared the four plots seen below in lab, but forgot to label the axes. A classmate later told him that the reaction was second order in NO and first order in O2. Not wanting to get a bad grade in the class, the student sets out to label the axes on the graphs.
![<strong>Exhibit 18-5 The following question(s) pertain to the scenario below. A student performed a kinetic study of the reaction: 2NO(g) + O<sub>2</sub>(g) → 2NO<sub>2</sub>(g) The student prepared the four plots seen below in lab, but forgot to label the axes. A classmate later told him that the reaction was second order in NO and first order in O<sub>2</sub>. Not wanting to get a bad grade in the class, the student sets out to label the axes on the graphs. Refer to Exhibit 18-5. Which graph corresponds to plotting ln[O<sub>2</sub>] vs. time?</strong> A) top left B) top right C) bottom left D) bottom right E) none of the above](https://storage.examlex.com/TB10703/11ed398b_834b_bf10_a786_abd83866d648_TB10703_00.jpg) Refer to Exhibit 18-5. Which graph corresponds to plotting ln[O2] vs. time?
Refer to Exhibit 18-5. Which graph corresponds to plotting ln[O2] vs. time?A) top left
B) top right
C) bottom left
D) bottom right
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
30
Exhibit 18-5
The following question(s) pertain to the scenario below.
A student performed a kinetic study of the reaction:
2NO(g) + O2(g) → 2NO2(g)
The student prepared the four plots seen below in lab, but forgot to label the axes. A classmate later told him that the reaction was second order in NO and first order in O2. Not wanting to get a bad grade in the class, the student sets out to label the axes on the graphs.![<strong>Exhibit 18-5 The following question(s) pertain to the scenario below. A student performed a kinetic study of the reaction: 2NO(g) + O<sub>2</sub>(g) → 2NO<sub>2</sub>(g) The student prepared the four plots seen below in lab, but forgot to label the axes. A classmate later told him that the reaction was second order in NO and first order in O<sub>2</sub>. Not wanting to get a bad grade in the class, the student sets out to label the axes on the graphs. Refer to Exhibit 18-5. Which graph corresponds to plotting 1/[NO] vs. time?</strong> A) top left B) top right C) bottom left D) bottom right E) none of the above](https://storage.examlex.com/TB10703/11ed398b_834b_e621_a786_3d2863815051_TB10703_00.jpg) Refer to Exhibit 18-5. Which graph corresponds to plotting 1/[NO] vs. time?
Refer to Exhibit 18-5. Which graph corresponds to plotting 1/[NO] vs. time?
A) top left
B) top right
C) bottom left
D) bottom right
E) none of the above
The following question(s) pertain to the scenario below.
A student performed a kinetic study of the reaction:
2NO(g) + O2(g) → 2NO2(g)
The student prepared the four plots seen below in lab, but forgot to label the axes. A classmate later told him that the reaction was second order in NO and first order in O2. Not wanting to get a bad grade in the class, the student sets out to label the axes on the graphs.
![<strong>Exhibit 18-5 The following question(s) pertain to the scenario below. A student performed a kinetic study of the reaction: 2NO(g) + O<sub>2</sub>(g) → 2NO<sub>2</sub>(g) The student prepared the four plots seen below in lab, but forgot to label the axes. A classmate later told him that the reaction was second order in NO and first order in O<sub>2</sub>. Not wanting to get a bad grade in the class, the student sets out to label the axes on the graphs. Refer to Exhibit 18-5. Which graph corresponds to plotting 1/[NO] vs. time?</strong> A) top left B) top right C) bottom left D) bottom right E) none of the above](https://storage.examlex.com/TB10703/11ed398b_834b_e621_a786_3d2863815051_TB10703_00.jpg) Refer to Exhibit 18-5. Which graph corresponds to plotting 1/[NO] vs. time?
Refer to Exhibit 18-5. Which graph corresponds to plotting 1/[NO] vs. time?A) top left
B) top right
C) bottom left
D) bottom right
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
31
The collision rate per unit volume in a mixture increases with
A) the collision cross section
B) the mass of the molecules
C) the concentration
D) B and C
E) A and C
A) the collision cross section
B) the mass of the molecules
C) the concentration
D) B and C
E) A and C

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
32
For two colliding molecules with respective radii r1 and r2, the impact parameter, b, will always be
A) greater than the smaller of the two radii
B) greater than the larger of the two radii
C) equal to the sum of the two radii
D) greater than the sum of the two radii
E) less than the sum of the two radii
A) greater than the smaller of the two radii
B) greater than the larger of the two radii
C) equal to the sum of the two radii
D) greater than the sum of the two radii
E) less than the sum of the two radii

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
33
Rate constants depend on
A) the collision cross section
B) the relative speed and orientation of impact
C) the probability that a collision will have sufficient energy to cause a reaction
D) All of the above
E) only B and C
A) the collision cross section
B) the relative speed and orientation of impact
C) the probability that a collision will have sufficient energy to cause a reaction
D) All of the above
E) only B and C

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck


