Deck 17: Electrochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/33
Play
Full screen (f)
Deck 17: Electrochemistry
1
Which of the following is true about an electrochemical system that has reached chemical equilibrium:
A) Ecell = 0
B) E°cell = 0
C) the concentration of the cathode ions equals that of the anode ions
D) a and b
E) a and c
A) Ecell = 0
B) E°cell = 0
C) the concentration of the cathode ions equals that of the anode ions
D) a and b
E) a and c
A
2
An ampere is equal to:
A) V·C-1
B) J·C-1
C) C·s-1
D) 96485 electrons
E) None of the above
A) V·C-1
B) J·C-1
C) C·s-1
D) 96485 electrons
E) None of the above
C
3
The faraday is equal to:
A) R·T·n-1
B) I·t
C) 96485 electrons
D) NA·1.602×10-19 C
E) None of the above
A) R·T·n-1
B) I·t
C) 96485 electrons
D) NA·1.602×10-19 C
E) None of the above
D
4
In a galvanic cell, oxidation always takes place in the:
A) cathode cell
B) anode cell
C) unable to answer without knowing sign of external potential
D) cell with the more positive reduction potential
A) cathode cell
B) anode cell
C) unable to answer without knowing sign of external potential
D) cell with the more positive reduction potential

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is a true statement
A) reduction takes place at the cathode
B) reduction takes place at the anode
C) reduction takes place in the cell with the more positive reduction potential
D) a and c
E) b and c
A) reduction takes place at the cathode
B) reduction takes place at the anode
C) reduction takes place in the cell with the more positive reduction potential
D) a and c
E) b and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
6
Exhibit 17-1
The following question(s) pertain to the electrochemical system pictured below. Cell A contains an aluminum electrode and a 1 molar solution of Al(NO3)3. Cell B contains an iron electrode and a 1 molar solution of Fe(NO3)2.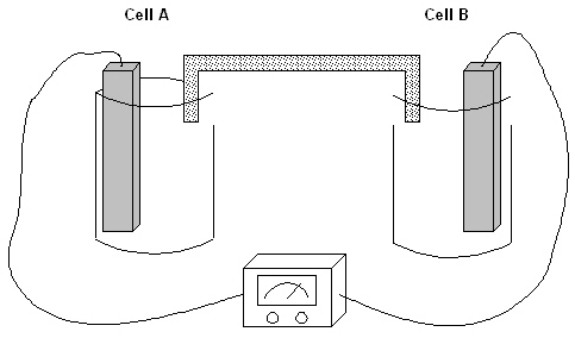 Refer to Exhibit 17-1. In which directions will electrons flow?
Refer to Exhibit 17-1. In which directions will electrons flow?
A) From A to B
B) From B to A
C) No electrons will actually flow between the cells
D) From cathode to anode
The following question(s) pertain to the electrochemical system pictured below. Cell A contains an aluminum electrode and a 1 molar solution of Al(NO3)3. Cell B contains an iron electrode and a 1 molar solution of Fe(NO3)2.
 Refer to Exhibit 17-1. In which directions will electrons flow?
Refer to Exhibit 17-1. In which directions will electrons flow?A) From A to B
B) From B to A
C) No electrons will actually flow between the cells
D) From cathode to anode

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
7
Exhibit 17-1
The following question(s) pertain to the electrochemical system pictured below. Cell A contains an aluminum electrode and a 1 molar solution of Al(NO3)3. Cell B contains an iron electrode and a 1 molar solution of Fe(NO3)2.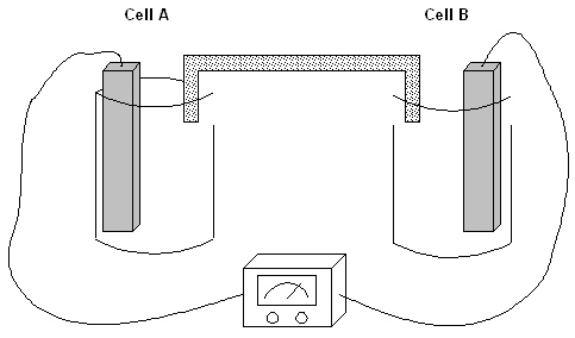 Refer to Exhibit 17-1. Which cell above is the cathode?
Refer to Exhibit 17-1. Which cell above is the cathode?
A) Cell A
B) Cell B
C) Not enough data to answer the question
D) The cell with the more negative reduction potential
E) None of the above
The following question(s) pertain to the electrochemical system pictured below. Cell A contains an aluminum electrode and a 1 molar solution of Al(NO3)3. Cell B contains an iron electrode and a 1 molar solution of Fe(NO3)2.
 Refer to Exhibit 17-1. Which cell above is the cathode?
Refer to Exhibit 17-1. Which cell above is the cathode?A) Cell A
B) Cell B
C) Not enough data to answer the question
D) The cell with the more negative reduction potential
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
8
Exhibit 17-1
The following question(s) pertain to the electrochemical system pictured below. Cell A contains an aluminum electrode and a 1 molar solution of Al(NO3)3. Cell B contains an iron electrode and a 1 molar solution of Fe(NO3)2.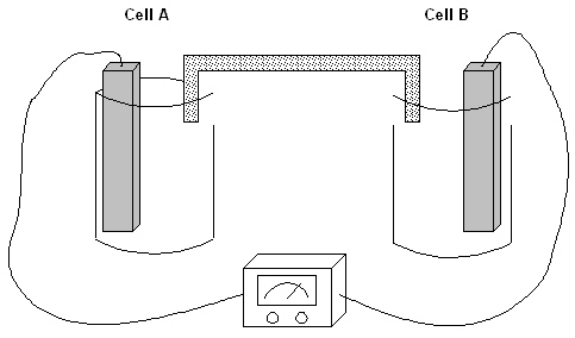 Refer to Exhibit 17-1. What is the value of E°cell?
Refer to Exhibit 17-1. What is the value of E°cell?
A) Cell A
B) Cell B
C) Not enough data to answer the question
D) The cell with the more negative reduction potential
E) None of the above
The following question(s) pertain to the electrochemical system pictured below. Cell A contains an aluminum electrode and a 1 molar solution of Al(NO3)3. Cell B contains an iron electrode and a 1 molar solution of Fe(NO3)2.
 Refer to Exhibit 17-1. What is the value of E°cell?
Refer to Exhibit 17-1. What is the value of E°cell?A) Cell A
B) Cell B
C) Not enough data to answer the question
D) The cell with the more negative reduction potential
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
9
Exhibit 17-1
The following question(s) pertain to the electrochemical system pictured below. Cell A contains an aluminum electrode and a 1 molar solution of Al(NO3)3. Cell B contains an iron electrode and a 1 molar solution of Fe(NO3)2.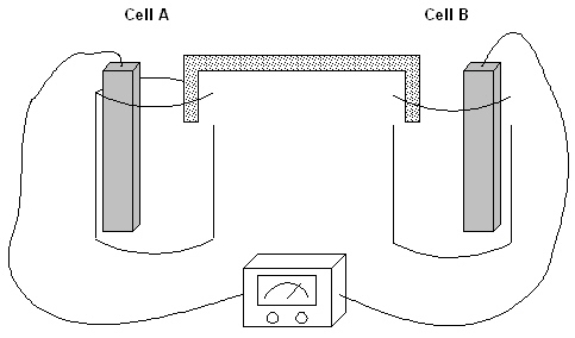 Refer to Exhibit 17-1. At the instant the two cells are connected, what is the value of Ecell?
Refer to Exhibit 17-1. At the instant the two cells are connected, what is the value of Ecell?
A) -E°cell
B) E°cell
C) Not enough data to answer the question
D) 0 V
E) None of the above
The following question(s) pertain to the electrochemical system pictured below. Cell A contains an aluminum electrode and a 1 molar solution of Al(NO3)3. Cell B contains an iron electrode and a 1 molar solution of Fe(NO3)2.
 Refer to Exhibit 17-1. At the instant the two cells are connected, what is the value of Ecell?
Refer to Exhibit 17-1. At the instant the two cells are connected, what is the value of Ecell?A) -E°cell
B) E°cell
C) Not enough data to answer the question
D) 0 V
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
10
Exhibit 17-1
The following question(s) pertain to the electrochemical system pictured below. Cell A contains an aluminum electrode and a 1 molar solution of Al(NO3)3. Cell B contains an iron electrode and a 1 molar solution of Fe(NO3)2.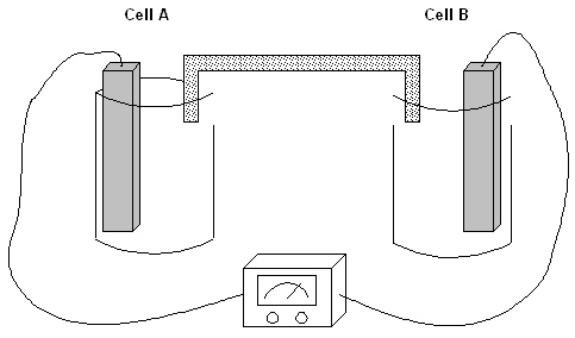 Refer to Exhibit 17-1. Which electrode is gaining mass as the reaction proceeds?
Refer to Exhibit 17-1. Which electrode is gaining mass as the reaction proceeds?
A) Neither, they just act as a conduit for the electrons
B) Both gain mass in this particular reaction
C) Al
D) Fe
The following question(s) pertain to the electrochemical system pictured below. Cell A contains an aluminum electrode and a 1 molar solution of Al(NO3)3. Cell B contains an iron electrode and a 1 molar solution of Fe(NO3)2.
 Refer to Exhibit 17-1. Which electrode is gaining mass as the reaction proceeds?
Refer to Exhibit 17-1. Which electrode is gaining mass as the reaction proceeds?A) Neither, they just act as a conduit for the electrons
B) Both gain mass in this particular reaction
C) Al
D) Fe

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
11
Exhibit 17-1
The following question(s) pertain to the electrochemical system pictured below. Cell A contains an aluminum electrode and a 1 molar solution of Al(NO3)3. Cell B contains an iron electrode and a 1 molar solution of Fe(NO3)2.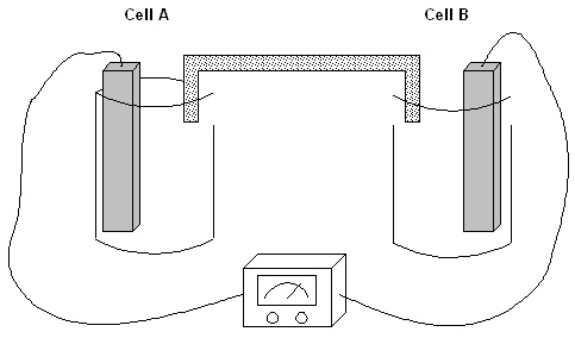 Refer to Exhibit 17-1. Which of the following is the chemical equation for the cell reaction that is spontaneous as written?
Refer to Exhibit 17-1. Which of the following is the chemical equation for the cell reaction that is spontaneous as written?
A) 3Fe(NO3)2(aq) + 2Al(NO3)3(aq) + 6e- → 2Al(s) + 3Fe(s) + 5NO3-(aq)
B) 3Fe(s) + 2Al3+ → 2Al(s) + 3Fe2+
C) Fe(s) + Al3+ → Al(s) + Fe3+
D) Al(s) + 2Fe3+ → 2Fe(s) + 3Al3+ (aq)
E) None of the above
The following question(s) pertain to the electrochemical system pictured below. Cell A contains an aluminum electrode and a 1 molar solution of Al(NO3)3. Cell B contains an iron electrode and a 1 molar solution of Fe(NO3)2.
 Refer to Exhibit 17-1. Which of the following is the chemical equation for the cell reaction that is spontaneous as written?
Refer to Exhibit 17-1. Which of the following is the chemical equation for the cell reaction that is spontaneous as written?A) 3Fe(NO3)2(aq) + 2Al(NO3)3(aq) + 6e- → 2Al(s) + 3Fe(s) + 5NO3-(aq)
B) 3Fe(s) + 2Al3+ → 2Al(s) + 3Fe2+
C) Fe(s) + Al3+ → Al(s) + Fe3+
D) Al(s) + 2Fe3+ → 2Fe(s) + 3Al3+ (aq)
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
12
Exhibit 17-1
The following question(s) pertain to the electrochemical system pictured below. Cell A contains an aluminum electrode and a 1 molar solution of Al(NO3)3. Cell B contains an iron electrode and a 1 molar solution of Fe(NO3)2.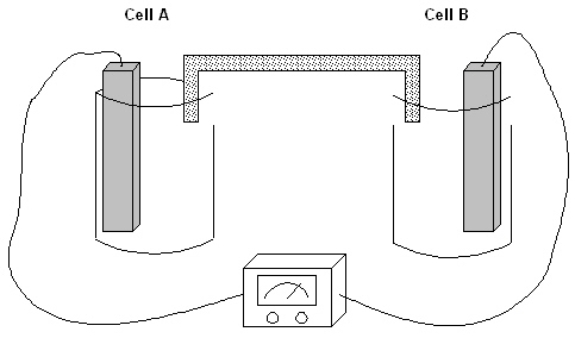 Refer to Exhibit 17-1. Which of the following represents the cell using standard cell notation?
Refer to Exhibit 17-1. Which of the following represents the cell using standard cell notation?
A) Al|Al3+||Fe|Fe2+
B) Al3+|Al||Fe|Fe2+
C) Al|Al3+||Fe2+|Fe
D) Fe|Fe3+||Al|Al3+
E) Fe2+|Fe|Al|Al3+
The following question(s) pertain to the electrochemical system pictured below. Cell A contains an aluminum electrode and a 1 molar solution of Al(NO3)3. Cell B contains an iron electrode and a 1 molar solution of Fe(NO3)2.
 Refer to Exhibit 17-1. Which of the following represents the cell using standard cell notation?
Refer to Exhibit 17-1. Which of the following represents the cell using standard cell notation?A) Al|Al3+||Fe|Fe2+
B) Al3+|Al||Fe|Fe2+
C) Al|Al3+||Fe2+|Fe
D) Fe|Fe3+||Al|Al3+
E) Fe2+|Fe|Al|Al3+

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
13
Exhibit 17-2
The following question(s) pertain to an electrochemical system in which a 1.5 V battery supplies a current of 0.5 amps for 18 hours to an iron electrode immersed in a solution of nickel nitrate.
Refer to Exhibit 17-2. What is the total charge that flows to the iron electrode?
A) 13.5 C
B) 81.0 C
C) 48600 C
D) 96485 C
E) None of the Above
The following question(s) pertain to an electrochemical system in which a 1.5 V battery supplies a current of 0.5 amps for 18 hours to an iron electrode immersed in a solution of nickel nitrate.
Refer to Exhibit 17-2. What is the total charge that flows to the iron electrode?
A) 13.5 C
B) 81.0 C
C) 48600 C
D) 96485 C
E) None of the Above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
14
Exhibit 17-2
The following question(s) pertain to an electrochemical system in which a 1.5 V battery supplies a current of 0.5 amps for 18 hours to an iron electrode immersed in a solution of nickel nitrate.
Refer to Exhibit 17-2. What is the amount of electrical work done by the battery?
A) -72900 J
B) 72900 J
C) 122 J
D) -122 J
E) Not enough information provided
The following question(s) pertain to an electrochemical system in which a 1.5 V battery supplies a current of 0.5 amps for 18 hours to an iron electrode immersed in a solution of nickel nitrate.
Refer to Exhibit 17-2. What is the amount of electrical work done by the battery?
A) -72900 J
B) 72900 J
C) 122 J
D) -122 J
E) Not enough information provided

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
15
Exhibit 17-2
The following question(s) pertain to an electrochemical system in which a 1.5 V battery supplies a current of 0.5 amps for 18 hours to an iron electrode immersed in a solution of nickel nitrate.
Refer to Exhibit 17-2. How many moles of electrons flow through the wire?
A) 96485
B) 0.756
C) 0.504
D) 1.40×10-4
E) None of the above
The following question(s) pertain to an electrochemical system in which a 1.5 V battery supplies a current of 0.5 amps for 18 hours to an iron electrode immersed in a solution of nickel nitrate.
Refer to Exhibit 17-2. How many moles of electrons flow through the wire?
A) 96485
B) 0.756
C) 0.504
D) 1.40×10-4
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
16
Exhibit 17-2
The following question(s) pertain to an electrochemical system in which a 1.5 V battery supplies a current of 0.5 amps for 18 hours to an iron electrode immersed in a solution of nickel nitrate.
Refer to Exhibit 17-2. The iron electrode is
A) gaining mass.
B) losing mass.
C) being oxidized.
D) b and c
E) none of the above
The following question(s) pertain to an electrochemical system in which a 1.5 V battery supplies a current of 0.5 amps for 18 hours to an iron electrode immersed in a solution of nickel nitrate.
Refer to Exhibit 17-2. The iron electrode is
A) gaining mass.
B) losing mass.
C) being oxidized.
D) b and c
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
17
Exhibit 17-2
The following question(s) pertain to an electrochemical system in which a 1.5 V battery supplies a current of 0.5 amps for 18 hours to an iron electrode immersed in a solution of nickel nitrate.
Refer to Exhibit 17-2. If the iron electrode initially weighed 20.00 g, what is the mass after the flow of current is stopped?
A) 34.79 g
B) 34.07 g
C) 49.57 g
D) 5.22 g
E) 5.93 g
The following question(s) pertain to an electrochemical system in which a 1.5 V battery supplies a current of 0.5 amps for 18 hours to an iron electrode immersed in a solution of nickel nitrate.
Refer to Exhibit 17-2. If the iron electrode initially weighed 20.00 g, what is the mass after the flow of current is stopped?
A) 34.79 g
B) 34.07 g
C) 49.57 g
D) 5.22 g
E) 5.93 g

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
18
Exhibit 17-3
The following question(s) pertain to the electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)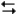 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)
Refer to Exhibit 17-3. What is the value of "n" used in the Nernst equation for the reaction above?
A) 1
B) 2
C) 3
D) Not enough data given to answer the question
E) None of the above
The following question(s) pertain to the electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)
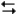 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)Refer to Exhibit 17-3. What is the value of "n" used in the Nernst equation for the reaction above?
A) 1
B) 2
C) 3
D) Not enough data given to answer the question
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
19
Exhibit 17-3
The following question(s) pertain to the electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)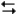 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)
Refer to Exhibit 17-3. What is the value of E°cell for the reaction above?
A) .770
B) .296
C) .7996
D) None of the above
The following question(s) pertain to the electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)
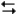 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)Refer to Exhibit 17-3. What is the value of E°cell for the reaction above?
A) .770
B) .296
C) .7996
D) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
20
Exhibit 17-3
The following question(s) pertain to the electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)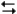 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)
Refer to Exhibit 17-3. What is the value of the equilibrium constant, K, for the reaction above at 25°C?
A) 1.15
B) 3.17
C) .142
D) Not enough information is given to answer the question
E) None of the above
The following question(s) pertain to the electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)
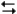 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)Refer to Exhibit 17-3. What is the value of the equilibrium constant, K, for the reaction above at 25°C?
A) 1.15
B) 3.17
C) .142
D) Not enough information is given to answer the question
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
21
Exhibit 17-3
The following question(s) pertain to the electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)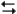 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)
Refer to Exhibit 17-3. What is the value of the reaction quotient (Q) for this reaction?
A) 4.04
B) 1.11
C) 0.727
D) 0.248
E) Not enough information is given to answer the question
The following question(s) pertain to the electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)
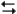 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)Refer to Exhibit 17-3. What is the value of the reaction quotient (Q) for this reaction?
A) 4.04
B) 1.11
C) 0.727
D) 0.248
E) Not enough information is given to answer the question

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
22
Exhibit 17-3
The following question(s) pertain to the electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)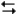 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)
Refer to Exhibit 17-3. Do you expect the silver electrode to gain mass or lose mass as the reaction approaches equilibrium?
A) Gain mass
B) Lose mass
C) Neither, solids do not appear in the equilibrium constant expression
D) Not enough information is given to answer the question
E) None of the above
The following question(s) pertain to the electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)
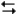 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)Refer to Exhibit 17-3. Do you expect the silver electrode to gain mass or lose mass as the reaction approaches equilibrium?
A) Gain mass
B) Lose mass
C) Neither, solids do not appear in the equilibrium constant expression
D) Not enough information is given to answer the question
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
23
Exhibit 17-3
The following question(s) pertain to the electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)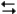 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)
Refer to Exhibit 17-3. What will be the concentration of Fe3+ when the reaction reaches equilibrium?
A) 0.183 M
B) 0.217 M
C) 0.112 M
D) 0.290 M
E) None of the above
The following question(s) pertain to the electrochemical reaction below at 25°C.
Ag+(0.275 M) + Fe2+(0.180 M)
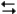 Fe3+(0.200 M) + Ag(s)
Fe3+(0.200 M) + Ag(s)Refer to Exhibit 17-3. What will be the concentration of Fe3+ when the reaction reaches equilibrium?
A) 0.183 M
B) 0.217 M
C) 0.112 M
D) 0.290 M
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
24
Exhibit 17-4
The following question(s) pertain to the electrochemical reaction below at 25°C written in standard cell notation.
Pt|H2 (1 atm)|H+(pH=1)||Cd2+(0.240 M)|Cd
Refer to Exhibit 17-4. What is the value of E°cell
A) -0.403
B) 0.403
C) 0.805
D) 0.201
E) None of the above
The following question(s) pertain to the electrochemical reaction below at 25°C written in standard cell notation.
Pt|H2 (1 atm)|H+(pH=1)||Cd2+(0.240 M)|Cd
Refer to Exhibit 17-4. What is the value of E°cell
A) -0.403
B) 0.403
C) 0.805
D) 0.201
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
25
Exhibit 17-4
The following question(s) pertain to the electrochemical reaction below at 25°C written in standard cell notation.
Pt|H2 (1 atm)|H+(pH=1)||Cd2+(0.240 M)|Cd
Refer to Exhibit 17-4. Which of the following is the chemical equation corresponding to the standard cell notation above?
A) Cd(s) + H2(g) → Cd2+(aq) + H+(aq)
B) Cd2+(aq) + H+(aq) → Cd(s) + H2(g)
C) Cd2+(aq) + H2(g) → H+(aq) + H+(aq)
D) H+(aq) + H+(aq) → Cd2+(aq) + H2(g)
E) None of the above
The following question(s) pertain to the electrochemical reaction below at 25°C written in standard cell notation.
Pt|H2 (1 atm)|H+(pH=1)||Cd2+(0.240 M)|Cd
Refer to Exhibit 17-4. Which of the following is the chemical equation corresponding to the standard cell notation above?
A) Cd(s) + H2(g) → Cd2+(aq) + H+(aq)
B) Cd2+(aq) + H+(aq) → Cd(s) + H2(g)
C) Cd2+(aq) + H2(g) → H+(aq) + H+(aq)
D) H+(aq) + H+(aq) → Cd2+(aq) + H2(g)
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
26
Exhibit 17-4
The following question(s) pertain to the electrochemical reaction below at 25°C written in standard cell notation.
Pt|H2 (1 atm)|H+(pH=1)||Cd2+(0.240 M)|Cd
Refer to Exhibit 17-4. What is the value of Ecell ?
A) 0.414 V
B) 0.443 V
C) 0.662 V
D) 0.425 V
E) None of the above
The following question(s) pertain to the electrochemical reaction below at 25°C written in standard cell notation.
Pt|H2 (1 atm)|H+(pH=1)||Cd2+(0.240 M)|Cd
Refer to Exhibit 17-4. What is the value of Ecell ?
A) 0.414 V
B) 0.443 V
C) 0.662 V
D) 0.425 V
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
27
A Zn|Zn2+||Co2+|Co galvanic cell has a standard potential E°cell=0.48 V. Calculate the free energy change at 25°C for every 1.00 g of Zn (M=65.37 g/mol) that is converted to Zn2+ at the anode, assuming that all concentrations remain at their standard values of 1.00 M throughout the process.
A) -93 kJ
B) -46 kJ
C) -1.42 kJ
D) -0.71 kJ
E) None of the above
A) -93 kJ
B) -46 kJ
C) -1.42 kJ
D) -0.71 kJ
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
28
The standard potential of a galvanic cell is
A) the voltage required by the cell to function.
B) the voltage produced by the cell.
C) the voltage required by the cell when all reactants and products are in their standard states.
D) the voltage produced by the cell when all reactants and products are in their standard states.
E) None of the above
A) the voltage required by the cell to function.
B) the voltage produced by the cell.
C) the voltage required by the cell when all reactants and products are in their standard states.
D) the voltage produced by the cell when all reactants and products are in their standard states.
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
29
For the half-reaction, Br2(l) + 2e- → 2Br-(aq), the standard reduction potential is 1.065 V. Thus for the half-reaction - 2Br2(l) + 4e- → 4Br-(aq),
A) E°=1.065 V
B) E°=0.532 V
C) E°=2.130 V
D) Not enough information is given to answer the question.
E) None of the above
A) E°=1.065 V
B) E°=0.532 V
C) E°=2.130 V
D) Not enough information is given to answer the question.
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
30
If the reference standard hydrogen half-cell were assigned a value of E°= -0.10 V instead of its customary value of 0.00 V, then the cell potential DE° for any chosen electrochemical cell would
A) increase by 0.10 V
B) decrease by 0.10 V
C) increase by 0.20 V
D) increase by 0.20 V
E) remain unchanged
A) increase by 0.10 V
B) decrease by 0.10 V
C) increase by 0.20 V
D) increase by 0.20 V
E) remain unchanged

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
31
As a battery discharges over time, the work done by the battery __________ in magnitude with a sign that is __________.
A) decreases, negative
B) decreases, positive
C) increases, negative
D) increases, positive
E) more information needed to answer the problem
A) decreases, negative
B) decreases, positive
C) increases, negative
D) increases, positive
E) more information needed to answer the problem

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
32
In the following equation 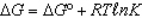
A)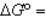 0
0
B)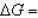 0
0
C) K = 1
D)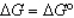
E) All of the above
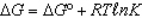
A)
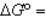 0
0B)
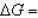 0
0C) K = 1
D)
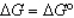
E) All of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following would be a good sacrificial electrode to protect an iron boat from corrosion?
A) Nickel
B) Chromium
C) Aluminum
D) B and C
E) None of the above
A) Nickel
B) Chromium
C) Aluminum
D) B and C
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck


