Deck 13: Spontaneous Processes and Thermodynamic Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/33
Play
Full screen (f)
Deck 13: Spontaneous Processes and Thermodynamic Equilibrium
1
Entropy has units of J·K-1, therefore the Boltzmann constant has units equivalent to
A) J·K-1
B) R
C) R/NA
D) a and b
E) a and c
A) J·K-1
B) R
C) R/NA
D) a and b
E) a and c
E
2
Molar entropies have units of
A) J·K-1
B) J·mol·K-1
C) J·mol-1·K-1
D) TDH
E) b and d
A) J·K-1
B) J·mol·K-1
C) J·mol-1·K-1
D) TDH
E) b and d
C
3
In going from a solid to a liquid, the entropy of a system will always
A) increase.
B) decrease.
C) remain constant so long as the phase transition takes place at a constant temperature.
D) be equal to the logarithm of the new number of microstates available.
A) increase.
B) decrease.
C) remain constant so long as the phase transition takes place at a constant temperature.
D) be equal to the logarithm of the new number of microstates available.
A
4
The evaporation of a liquid is
A) always spontaneous.
B) always associated with an increase in entropy.
C) a and b
D) None of the above
A) always spontaneous.
B) always associated with an increase in entropy.
C) a and b
D) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
5
An increase in entropy for a closed system is always associated with
A) an increase in the number of microstates available to the system.
B) an increase in the number of molecules in the system.
C) a and b
D) None of the above
A) an increase in the number of microstates available to the system.
B) an increase in the number of molecules in the system.
C) a and b
D) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
6
For an isentropic process
A) q=0
B) w=0
C) DS=0
D) a and c
E) b and c
A) q=0
B) w=0
C) DS=0
D) a and c
E) b and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
7
An element has a standard state molar entropy that is always
A) negative
B) zero
C) positive
D) not enough data to answer the question
A) negative
B) zero
C) positive
D) not enough data to answer the question

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
8
An element has a standard state molar Gibbs free energy that is always
A) negative
B) zero
C) positive
D) not enough data to answer the question
A) negative
B) zero
C) positive
D) not enough data to answer the question

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following has a standard state Gibbs free energy equal to zero?
A) Br2 (l)
B) I2 (g)
C) C (s, diamond)
D) a and b
E) b and c
A) Br2 (l)
B) I2 (g)
C) C (s, diamond)
D) a and b
E) b and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following has a standard state entropy equal to zero?
A) Br2 (l)
B) I2 (g)
C) C (s, diamond)
D) a and b
E) b ad c
F) none of the above
A) Br2 (l)
B) I2 (g)
C) C (s, diamond)
D) a and b
E) b ad c
F) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is true for an equilibrium process?
A) DSuniv = 0
B) DSuniv < 0
C) DSuniv > 0
D) DSsys = DSsurr
E) none of the above
A) DSuniv = 0
B) DSuniv < 0
C) DSuniv > 0
D) DSsys = DSsurr
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is true for a spontaneous process?
A) DSuniv = 0
B) DSuniv < 0
C) DSuniv > 0
D) DSsys = DSsurr
E) none of the above
A) DSuniv = 0
B) DSuniv < 0
C) DSuniv > 0
D) DSsys = DSsurr
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following equations are true for a system at thermodynamic equilibrium?
A) DH=TDS
B) DG=0
C) DH=-TDS
D) a and b
E) b and c
A) DH=TDS
B) DG=0
C) DH=-TDS
D) a and b
E) b and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
14
A process can be spontaneous at low temperature but nonspontaneous at high temperature if
A) both DH and DS are positive
B) both DH and DS are negative
C) DH is positive and DS is negative
D) DH is negative and DS is positive
E) this can never happen
A) both DH and DS are positive
B) both DH and DS are negative
C) DH is positive and DS is negative
D) DH is negative and DS is positive
E) this can never happen

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
15
Predict the sign of the entropy change for the following reaction
PCl3(g) + Cl2(g) → PCl5(g)
A) positive
B) negative
C) zero
D) more data is needed to answer this question
PCl3(g) + Cl2(g) → PCl5(g)
A) positive
B) negative
C) zero
D) more data is needed to answer this question

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
16
Predict the sign of the free energy change for the following reaction
PCl3(g) + Cl2(g) → PCl5(g)
A) positive
B) negative
C) zero
D) more data is needed to answer this question
PCl3(g) + Cl2(g) → PCl5(g)
A) positive
B) negative
C) zero
D) more data is needed to answer this question

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
17
Predict the sign of the entropy change for the following reaction
H2(g) + I2(g) → 2HI(g)
A) positive
B) negative
C) zero
D) more data is needed to answer this question
H2(g) + I2(g) → 2HI(g)
A) positive
B) negative
C) zero
D) more data is needed to answer this question

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
18
As temperature increases, the value of DGrxn for the following reaction will become
N2(g) + 3H2(g) → 2NH3(g)
A) More positive
B) More negative
C) Not change
D) more data is needed to answer this question
N2(g) + 3H2(g) → 2NH3(g)
A) More positive
B) More negative
C) Not change
D) more data is needed to answer this question

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
19
Calculate DS°rxn for the following
NH3(g) + HCl(g) → NH4Cl(s)
A) 284.5 J·K-1
B) -284.5 J·K-1
C) -203.5 J·K-1
D) 89.1 J·K-1
NH3(g) + HCl(g) → NH4Cl(s)
A) 284.5 J·K-1
B) -284.5 J·K-1
C) -203.5 J·K-1
D) 89.1 J·K-1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
20
Calculate DS°rxn for the following
3NO2(g) + H2O(l) → 2HNO3(l) + NO(g)
A) 56.28 J·K-1
B) -56.28 J·K-1
C) 268.1 J·K-1
D) -268.1 J·K-1
3NO2(g) + H2O(l) → 2HNO3(l) + NO(g)
A) 56.28 J·K-1
B) -56.28 J·K-1
C) 268.1 J·K-1
D) -268.1 J·K-1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
21
Acetone boils at 53°C. Using Trouton's rule, estimate acetone's molar enthalpy of vaporization.
A) 36.8 kJ
B) -16.7 kJ
C) 28.7 kJ
D) 4.66 kJ
A) 36.8 kJ
B) -16.7 kJ
C) 28.7 kJ
D) 4.66 kJ

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
22
Exhibit 13-1
The following question(s) pertain to the reaction CCl4(l) → CCl4(g).
Refer to Exhibit 13-1. What is the value of DS°vap?
A) 93.3 J·K-1
B) -93.3 J·K-1
C) 32.5 J·K-1
D) -32.5 J·K-1
E) None of the above
The following question(s) pertain to the reaction CCl4(l) → CCl4(g).
Refer to Exhibit 13-1. What is the value of DS°vap?
A) 93.3 J·K-1
B) -93.3 J·K-1
C) 32.5 J·K-1
D) -32.5 J·K-1
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
23
Exhibit 13-1
The following question(s) pertain to the reaction CCl4(l) → CCl4(g).
Refer to Exhibit 13-1. What is value of DH°vap?
A) 93.3 kJ
B) -93.3 kJ
C) 32.5 kJ
D) -32.5 kJ
The following question(s) pertain to the reaction CCl4(l) → CCl4(g).
Refer to Exhibit 13-1. What is value of DH°vap?
A) 93.3 kJ
B) -93.3 kJ
C) 32.5 kJ
D) -32.5 kJ

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
24
Exhibit 13-1
The following question(s) pertain to the reaction CCl4(l) → CCl4(g).
Refer to Exhibit 13-1. What is the predicted boiling point of CCl4?
A) 85°C
B) 2400°C
C) 93°C
D) 113°C
E) None of the above
The following question(s) pertain to the reaction CCl4(l) → CCl4(g).
Refer to Exhibit 13-1. What is the predicted boiling point of CCl4?
A) 85°C
B) 2400°C
C) 93°C
D) 113°C
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
25
Exhibit 13-1
The following question(s) pertain to the reaction CCl4(l) → CCl4(g).
Refer to Exhibit 13-1. Using Trouton's rule, what is the approximate boiling point of CCl4?
A) 96°C
B) 787°C
C) 85°C
D) 110°C
E) None of the above
The following question(s) pertain to the reaction CCl4(l) → CCl4(g).
Refer to Exhibit 13-1. Using Trouton's rule, what is the approximate boiling point of CCl4?
A) 96°C
B) 787°C
C) 85°C
D) 110°C
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
26
The process I2(s) → I2(g) is spontaneous at 25°C?

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
27
Can the reaction C(s, graphite) → C(s, diamond) ever be spontaneous?

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
28
Exhibit 13-2
The following question(s) pertain to the reaction 2NO2(g) → N2O4(g).
Refer to Exhibit 13-2. Is this reaction spontaneous at 25°C?
The following question(s) pertain to the reaction 2NO2(g) → N2O4(g).
Refer to Exhibit 13-2. Is this reaction spontaneous at 25°C?

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
29
Exhibit 13-2
The following question(s) pertain to the reaction 2NO2(g) → N2O4(g).
Refer to Exhibit 13-2. As temperature increases, the value of DGrxn will?
A) become more positive
B) become more negative
C) asymptotically approach the value of DHrxn
D) equal the value of W
E) a and d
The following question(s) pertain to the reaction 2NO2(g) → N2O4(g).
Refer to Exhibit 13-2. As temperature increases, the value of DGrxn will?
A) become more positive
B) become more negative
C) asymptotically approach the value of DHrxn
D) equal the value of W
E) a and d

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
30
Exhibit 13-2
The following question(s) pertain to the reaction 2NO2(g) → N2O4(g).
Refer to Exhibit 13-2. At what temperature will the value of DGrxn = 0?
A) For this reaction, DG can never equal zero
B) 0 K
C) 96°C
D) 53°C
E) None of the above
The following question(s) pertain to the reaction 2NO2(g) → N2O4(g).
Refer to Exhibit 13-2. At what temperature will the value of DGrxn = 0?
A) For this reaction, DG can never equal zero
B) 0 K
C) 96°C
D) 53°C
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
31
Allotropic forms of an element that do not represent an element in its standard state will have
A)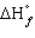 =
=
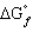 = 0
= 0
B)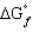 = 0,
= 0,
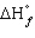 positive
positive
C)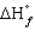 = 0,
= 0,
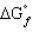 positive
positive
D) S°=0
E) None of the above
A)
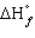 =
=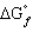 = 0
= 0B)
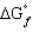 = 0,
= 0,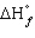 positive
positiveC)
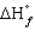 = 0,
= 0,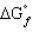 positive
positiveD) S°=0
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
32
A solid of mass m initially at T0 is heated to T1 at constant pressure where it proceeds to melt. Once all of the solid is in the liquid phase it is further heated to T2. If the solid and liquid have specific heat capacities of c 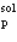 and c
and c 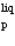 respectively, which equation below best represents the change in entropy for this system?
respectively, which equation below best represents the change in entropy for this system?
A)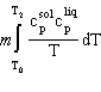
B)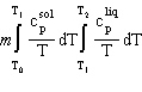
C)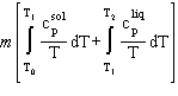
D)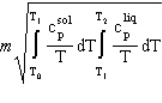
E) B and D
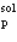 and c
and c 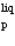 respectively, which equation below best represents the change in entropy for this system?
respectively, which equation below best represents the change in entropy for this system?A)
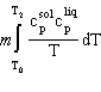
B)
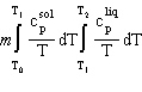
C)
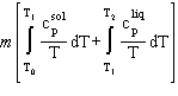
D)
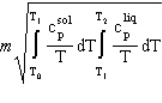
E) B and D

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
33
The constant that connects the entropy of a system to the number of microstates is
A) R
B)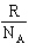
C) kB
D)
E) B and C
A) R
B)
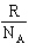
C) kB
D)

E) B and C

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck


