Deck 12: Thermodynamic Processes and Thermochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/33
Play
Full screen (f)
Deck 12: Thermodynamic Processes and Thermochemistry
1
In chemistry applications, the change in internal energy is equal to
A) q + w
B) q - w
C) the molar constant pressure heat capacity.
D) 0
E) None of the above
A) q + w
B) q - w
C) the molar constant pressure heat capacity.
D) 0
E) None of the above
A
2
By definition DUuniv equals
A) DUsys + DUsurr
B) -DUsys
C) 0
D) a and c
E) none of the above
A) DUsys + DUsurr
B) -DUsys
C) 0
D) a and c
E) none of the above
D
3
The change in enthalpy for a reaction is
A) Equal to the constant volume heat capacity
B) DU / (nRT)
C) DU + PDV
D) a and b
E) none of the above
A) Equal to the constant volume heat capacity
B) DU / (nRT)
C) DU + PDV
D) a and b
E) none of the above
E
4
A coffee cup calorimeter is best used to determine
A) qp
B) qv
C) DU
D) DH
E) a and d
A) qp
B) qv
C) DU
D) DH
E) a and d

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
5
A bomb calorimeter is best used to determine
A) qp
B) qv
C) DU
D) DH
E) a and d
F) b and c
A) qp
B) qv
C) DU
D) DH
E) a and d
F) b and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following are acceptable units for heat capacity?
A) J·°K·mol-1
B) J·°F-1·mol-1
C) J·°C-1·g-1
D) a and c
E) b and c
A) J·°K·mol-1
B) J·°F-1·mol-1
C) J·°C-1·g-1
D) a and c
E) b and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
7
When one combines a concentrated solution of a strong base with a concentrated solution of a strong acid, the beaker in which they are combined gets noticeably warmer. This reaction is therefore
A) endothermic
B) adiabatic
C) exothermic
D) isochoric
E) None of the above
A) endothermic
B) adiabatic
C) exothermic
D) isochoric
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
8
For an exothermic reaction which of the following must be true
A) DHsurr is positive
B) DHsurr is negative
C) DHsys is positive
D) q is positive
E) b and c
A) DHsurr is positive
B) DHsurr is negative
C) DHsys is positive
D) q is positive
E) b and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
9
For an endothermic reaction which of the following must be true
A) DHsurr is positive
B) DHsurr is negative
C) DHsys is positive
D) wsurr is positive
E) b and c
A) DHsurr is positive
B) DHsurr is negative
C) DHsys is positive
D) wsurr is positive
E) b and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
10
Isochoric processes take place
A) at constant molar concentration
B) at constant pressure
C) at constant volume
D) in adiabatic system where PV work is being done.
E) None of the above
A) at constant molar concentration
B) at constant pressure
C) at constant volume
D) in adiabatic system where PV work is being done.
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
11
For an isothermal process involving ideal gases
A) q = -w
B) DU > 0
C) q = 0
D) w = 0
E) a and b
A) q = -w
B) DU > 0
C) q = 0
D) w = 0
E) a and b

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
12
For a reversible isothermal process involving ideal gases
A) w = nRT ln
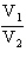
B) w =
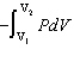
C) w = -nRT
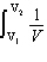
DV
D) b and c
E) all of the above
A) w = nRT ln
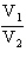
B) w =
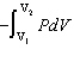
C) w = -nRT
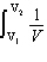
DV
D) b and c
E) all of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
13
For an adiabatic process involving ideal gases
A) q = w
B) w = 0
C) DU = 0
D) DU = w
E) a and d
A) q = w
B) w = 0
C) DU = 0
D) DU = w
E) a and d

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
14
The molar heat capacity of a substance
A) will always be greater than the specific heat capacity.
B) will always be less than the specific heat capacity.
C) will equal the specific heat capacity if one mole of substance is used.
D) equals zero if the substance is an ideal gas.
E) a and d
A) will always be greater than the specific heat capacity.
B) will always be less than the specific heat capacity.
C) will equal the specific heat capacity if one mole of substance is used.
D) equals zero if the substance is an ideal gas.
E) a and d

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is not a state function
A) U
B) P
C) V
D) H
E) PV
F) All of the above
G) None of the above
A) U
B) P
C) V
D) H
E) PV
F) All of the above
G) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
16
At 298 K, elements in their standard state have
A) Zero enthalpy
B) Zero internal energy
C) A constant volume molar heat capacity equal to the internal energy
D) A constant pressure molar heat capacity equal to the enthalpy
E) None of the above
A) Zero enthalpy
B) Zero internal energy
C) A constant volume molar heat capacity equal to the internal energy
D) A constant pressure molar heat capacity equal to the enthalpy
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
17
Copper has a specific heat capacity of 0.0920 cal·K-1·g-1 at 298 K. What is the molar heat capacity of copper in J·K-1·mol-1?
A) 22.6 J·K-1·mol-1
B) 24.5 J·K-1·mol-1
C) 1.40 J·K-1·mol-1
D) 3.46×10-4 J·K-1·mol-1
E) None of the above
A) 22.6 J·K-1·mol-1
B) 24.5 J·K-1·mol-1
C) 1.40 J·K-1·mol-1
D) 3.46×10-4 J·K-1·mol-1
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
18
Titanium has a specific heat capacity of 0.515 J·K-1·g-1 at 298 K. How much energy is required to raise the temperature of 2.00 moles of Titanium by 50°C?
A) 2.47 kJ
B) 10.5 kJ
C) 1.23 kJ
D) 9.43 kJ
E) None of the above
A) 2.47 kJ
B) 10.5 kJ
C) 1.23 kJ
D) 9.43 kJ
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
19
A 125.0 g piece of iron is heated to 400°C and placed in 300.0 g of water at 25°C. If the specific heat capacities of iron and water are 0.449 J·K-1·g-1 and 4.184 J·K-1·g-1 respectively, what will be the final temperature of the water?
A) 32°C
B) 41°C
C) 101°C
D) 383°C
E) None of the above
A) 32°C
B) 41°C
C) 101°C
D) 383°C
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
20
When combusted, which of the following will produce the hottest flame?
A) H3C-CH3
B) H2C=CH2
C) HCºCH
D) The flame temperature will be the same for all of these
A) H3C-CH3
B) H2C=CH2
C) HCºCH
D) The flame temperature will be the same for all of these

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
21
The decomposition of NO and NO2 to the elements proceeds via the following two reactions.
2NO(g) → N₂(g) + O₂(g) DH°rxn = -180 kJ
2NO₂(g) → N₂(g) + 2O₂(g) DH°rxn = -66.4 kJ
Calculate the enthalpy change for the reaction
2NO(g) + O2(g) → 2NO2(g)
A) 244 kJ
B) -244 kJ
C) -114 kJ
D) -293 kJ
E) none of the above
2NO(g) → N₂(g) + O₂(g) DH°rxn = -180 kJ
2NO₂(g) → N₂(g) + 2O₂(g) DH°rxn = -66.4 kJ
Calculate the enthalpy change for the reaction
2NO(g) + O2(g) → 2NO2(g)
A) 244 kJ
B) -244 kJ
C) -114 kJ
D) -293 kJ
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
22
Calculate the amount of energy necessary to raise the temperature of 1 cup of water at 23°C to steam at 105°C. The specific heat capacity of water is 4.184 J·K-1·g-1 and the specific heat capacity of steam is 4.215 J·K-1·g-1. The density of water can be assumed to be 1.00 g·ml-1. For water, DHvap=40.66 kJ·mol-1. 1 cup=0.2366 L
A) 81.2 kJ
B) 122 kJ
C) 534 kJ
D) 616 kJ
E) None of the above
A) 81.2 kJ
B) 122 kJ
C) 534 kJ
D) 616 kJ
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
23
Many residential microwave ovens operate at 900 watts. How long will it take a 900 watt microwave oven to raise the temperature of 1 cup of water at 23°C to steam at 105°C? The specific heat capacity of water is 4.184 J·K-1·g-1 and the specific heat capacity of steam is 4.215 J·K-1·g-1. The density of water can be assumed to be 1.00 g·ml-1. For water, DHvap=40.66 kJ·mol-1. 1 cup=0.2366, 1 W=1 J·s-1
A) 11.4 min
B) 38 seconds
C) 9.9 min
D) 1.5 min
E) None of the above
A) 11.4 min
B) 38 seconds
C) 9.9 min
D) 1.5 min
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
24
Sublimation is the process of changing phases directly from a solid to a gas. Calculate DH°sub for elemental iodine.
A) 62.44 kJ·mol-1
B) -62.44 kJ·mol-1
C) 39.98 kJ·mol-1
D) 19.36 kJ·mol-1
A) 62.44 kJ·mol-1
B) -62.44 kJ·mol-1
C) 39.98 kJ·mol-1
D) 19.36 kJ·mol-1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
25
Exhibit 12-1
The following question(s) pertain to the combustion of liquid ethanol (C2H5OH) to form carbon dioxide gas and water vapor.
Refer to Exhibit 12-1. For each mole of ethanol combusted, how many moles of carbon dioxide are formed?
A) 1
B) 2
C) 3
D) 4
The following question(s) pertain to the combustion of liquid ethanol (C2H5OH) to form carbon dioxide gas and water vapor.
Refer to Exhibit 12-1. For each mole of ethanol combusted, how many moles of carbon dioxide are formed?
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
26
Exhibit 12-1
The following question(s) pertain to the combustion of liquid ethanol (C2H5OH) to form carbon dioxide gas and water vapor.
Refer to Exhibit 12-1. What is DH°rxn for the combustion of ethanol?
A) -1367 kJ
B) -1235 kJ
C) -278 kJ
D) -913 kJ
The following question(s) pertain to the combustion of liquid ethanol (C2H5OH) to form carbon dioxide gas and water vapor.
Refer to Exhibit 12-1. What is DH°rxn for the combustion of ethanol?
A) -1367 kJ
B) -1235 kJ
C) -278 kJ
D) -913 kJ

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
27
Exhibit 12-1
The following question(s) pertain to the combustion of liquid ethanol (C2H5OH) to form carbon dioxide gas and water vapor.
Refer to Exhibit 12-1. How much heat is liberated when 800.0 g of ethanol is combusted at 25°C?
A) 5370 kJ
B) 21500 kJ
C) 23800 kJ
D) 1370 kJ
The following question(s) pertain to the combustion of liquid ethanol (C2H5OH) to form carbon dioxide gas and water vapor.
Refer to Exhibit 12-1. How much heat is liberated when 800.0 g of ethanol is combusted at 25°C?
A) 5370 kJ
B) 21500 kJ
C) 23800 kJ
D) 1370 kJ

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
28
Exhibit 12-2
The following question(s) pertain to the reaction between aluminum metal and iron (III) oxide to produce iron metal and aluminum oxide.
Refer to Exhibit 12-2. In the balanced stoichiometric equation, how many moles of aluminum metal have to react with each mole of iron (III) oxide?
A) 1
B) 2
C) 3
D) 4
The following question(s) pertain to the reaction between aluminum metal and iron (III) oxide to produce iron metal and aluminum oxide.
Refer to Exhibit 12-2. In the balanced stoichiometric equation, how many moles of aluminum metal have to react with each mole of iron (III) oxide?
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
29
Exhibit 12-2
The following question(s) pertain to the reaction between aluminum metal and iron (III) oxide to produce iron metal and aluminum oxide.
Refer to Exhibit 12-2. What is DH°rxn for this reaction?
A) -1680 kJ
B) 851 kJ
C) -851 kJ
D) -2530 kJ
The following question(s) pertain to the reaction between aluminum metal and iron (III) oxide to produce iron metal and aluminum oxide.
Refer to Exhibit 12-2. What is DH°rxn for this reaction?
A) -1680 kJ
B) 851 kJ
C) -851 kJ
D) -2530 kJ

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
30
Exhibit 12-2
The following question(s) pertain to the reaction between aluminum metal and iron (III) oxide to produce iron metal and aluminum oxide.
Refer to Exhibit 12-2. Calculate the enthalpy change when 200.0 grams of aluminum reacts with 300.0 grams of iron (III) oxide at 298 K.
A) -1600 kJ
B) -1520 kJ
C) -3141 kJ
D) 1520 kJ
The following question(s) pertain to the reaction between aluminum metal and iron (III) oxide to produce iron metal and aluminum oxide.
Refer to Exhibit 12-2. Calculate the enthalpy change when 200.0 grams of aluminum reacts with 300.0 grams of iron (III) oxide at 298 K.
A) -1600 kJ
B) -1520 kJ
C) -3141 kJ
D) 1520 kJ

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
31
Which molecule has the highest total number of internal degrees of freedom?
A) C6H6
B) C2H5OH
C) C2H5OCH3
D) A and C
E) Impossible to determine without structural information
A) C6H6
B) C2H5OH
C) C2H5OCH3
D) A and C
E) Impossible to determine without structural information

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
32
The constant pressure heat capacity (cp) generally increases with
A) the mass of the molecule
B) the internal degrees of freedom in the molecule
C) the number of atoms in the molecule
D) A and C
E) B and C
A) the mass of the molecule
B) the internal degrees of freedom in the molecule
C) the number of atoms in the molecule
D) A and C
E) B and C

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
33
The standard state of carbon as a free element is graphite. C60 is an allotropic form of carbon belonging to a class of structures known as fullerenes. 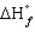 for C60 should be
for C60 should be
A) zero
B) positive
C) negative
D) equal to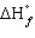
for the other allotropic forms of carbon
E) A and D
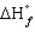 for C60 should be
for C60 should beA) zero
B) positive
C) negative
D) equal to
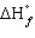
for the other allotropic forms of carbon
E) A and D

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck


