Deck 9: The Gaseous State
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/33
Play
Full screen (f)
Deck 9: The Gaseous State
1
Sodium bicarbonate, NaHCO3, reacts with HCl to form what gases?
A) H2
B) CO
C) CO2
D) NaCl
E) it does not react
A) H2
B) CO
C) CO2
D) NaCl
E) it does not react
C
2
Hydrogen peroxide, H2O2, decomposes into water and oxygen gas. How many moles of oxygen gas are formed from the decomposition of 1 mole of hydrogen peroxide?
A) 0
B) 0.5
C) 1
D) 1.5
E) 2
A) 0
B) 0.5
C) 1
D) 1.5
E) 2
B
3
10 L of a gas at constant pressure is initially at 25°C. If the temperature is increased to 50°C the volume of the gas will
A) increase by about 8%.
B) decrease by about 8%.
C) increase by 100%.
D) decrease by 100%.
E) stay the same.
A) increase by about 8%.
B) decrease by about 8%.
C) increase by 100%.
D) decrease by 100%.
E) stay the same.
A
4
Which of the following is not equal to 1 atm?
A) 760 Torr
B) 760 mm Hg (at 0°C)
C) 14.7 psi
D) 1 bar
E) 105 Pa
F) a & b
G) d & e
A) 760 Torr
B) 760 mm Hg (at 0°C)
C) 14.7 psi
D) 1 bar
E) 105 Pa
F) a & b
G) d & e

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
5
Ethanol has a density of 0.789 g cm-3. What is the pressure at the bottom of a 125 cm tall column of ethanol?
A) 9.66×102 Pa
B) 9.66×103 Pa
C) 9.66×104 Pa
D) 9.66×105 Pa
E) 9.66×108 Pa
A) 9.66×102 Pa
B) 9.66×103 Pa
C) 9.66×104 Pa
D) 9.66×105 Pa
E) 9.66×108 Pa

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
6
A gas has a temperature of 34.9°C and a volume of 2.8L. It is cooled at constant pressure and the new volume is 2.5 L. What is the new temperature of the gas?
A) 1.89°C
B) 11.6°C
C) 25.2°C
D) 31.2°C
E) 39.1°C
A) 1.89°C
B) 11.6°C
C) 25.2°C
D) 31.2°C
E) 39.1°C

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
7
Sodium metal reacts with water form hydrogen gas by the following unbalanced chemical equation
Na(s) + H2O(l) → NaOH(aq) + H2(g)
If 20 g of sodium are reacted with excess water at a temperature of 30°C and a pressure of 0.8 atm, what volume of H2 is produced?
A) 1.34 L
B) 13.5 L
C) 21.6 L
D) 26.7 L
E) 27.0
Na(s) + H2O(l) → NaOH(aq) + H2(g)
If 20 g of sodium are reacted with excess water at a temperature of 30°C and a pressure of 0.8 atm, what volume of H2 is produced?
A) 1.34 L
B) 13.5 L
C) 21.6 L
D) 26.7 L
E) 27.0

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
8
A cylinder of compressed He originally has a pressure of 20 atm and a volume 10 L at a temperature of 25°C. It is used to fill 10, 1 L balloons at a pressure of 0.99 atm at 25°C. After filling the balloons what is the pressure in the tank?
A) 12.0 atm
B) 17.2 atm
C) 18.0 atm
D) 19.0 atm
E) 19.8 atm
A) 12.0 atm
B) 17.2 atm
C) 18.0 atm
D) 19.0 atm
E) 19.8 atm

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
9
A balloon has a volume of 1.2 L at a pressure of 0.98 atm and a temperature of 25°C. It rises in the atmosphere to a point where the pressure of 0.28 atm and a temperature of -33°C. What is the volume of the balloon?
A) 0.42 L
B) 3.8 L
C) 5.6 L
D) 7.1 L
E) There is no way to know.
A) 0.42 L
B) 3.8 L
C) 5.6 L
D) 7.1 L
E) There is no way to know.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
10
At a temperature of 48.2°C a gas has volume of 6.8 gallons at a pressure of 282 psi. If the gas is compressed at constant temperature to a volume of 3 gallons, what is the pressure?
A) 124 psi
B) 226 psi
C) 308 psi
D) 639 psi
E) 785 psi
A) 124 psi
B) 226 psi
C) 308 psi
D) 639 psi
E) 785 psi

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
11
12.2 g of I2(s) is placed in an evacuated 15 L container and heated to 100°C. At this temperature all of the solid sublimes into vapor. What is the pressure in the container?
A) 2.65×103 Pa
B) 9.93×103 Pa
C) 1.82×104 Pa
D) 4.97×104 Pa
E) 8.23×105 Pa
A) 2.65×103 Pa
B) 9.93×103 Pa
C) 1.82×104 Pa
D) 4.97×104 Pa
E) 8.23×105 Pa

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
12
At a fixed temperature and pressure 4.5 L of F2(g) and 1.5 L of Cl2(g) react completely to from 3.0 L of a new gas. What is the formula of the new gas?
A) ClF
B) Cl2F2
C) ClF3
D) Cl2F6
E) Cl3F
A) ClF
B) Cl2F2
C) ClF3
D) Cl2F6
E) Cl3F

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
13
Ammonia can be formed from the reaction of hydrogen and nitrogen gas by the following reaction
N2(g) + 3H2(g) → 2NH3(g)
At 127°C an equilibrium mixture is found to have a partial pressure of 103 kPa for NH3, a partial pressure of 2030 kPa for N2, and a partial pressure of 10 kPa for H2. If the volume of the container is 10 L, how many moles of NH3 are there?
A) 0.31
B) 0.52
C) 0.98
D) 6.4
E) 20.0
N2(g) + 3H2(g) → 2NH3(g)
At 127°C an equilibrium mixture is found to have a partial pressure of 103 kPa for NH3, a partial pressure of 2030 kPa for N2, and a partial pressure of 10 kPa for H2. If the volume of the container is 10 L, how many moles of NH3 are there?
A) 0.31
B) 0.52
C) 0.98
D) 6.4
E) 20.0

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
14
Ammonia can be formed from the reaction of hydrogen and nitrogen gas by the following reaction
N2(g) + 3H2(g) → 2NH3(g)
At 127°C an equilibrium mixture is found to have a partial pressure of 103 kPa for NH3, a partial pressure of 2030 kPa for N2, and a partial pressure of 10 kPa for H2. What is the total pressure in the container?
A) 1.03 bar
B) 1.34 bar
C) 15.4 bar
D) 21.43 bar
E) it depends on the number of moles of gas
N2(g) + 3H2(g) → 2NH3(g)
At 127°C an equilibrium mixture is found to have a partial pressure of 103 kPa for NH3, a partial pressure of 2030 kPa for N2, and a partial pressure of 10 kPa for H2. What is the total pressure in the container?
A) 1.03 bar
B) 1.34 bar
C) 15.4 bar
D) 21.43 bar
E) it depends on the number of moles of gas

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
15
In the gas shift reaction carbon monoxide reacts with steam to from hydrogen gas and carbon dioxide
CO(g) + H2O(g) → H2(g) + CO2(g)
If a 10 L container initially has a partial pressure of 0.25 atm of CO and 1.35 atm of H2O at constant temperature of 1000 K. When the partial pressure of CO has dropped to 0.15 atm, what is the total pressure in the container?
A) 0.10 atm
B) 0.15 atm
C) 1.40 atm
D) 1.60 atm
E) 1.75 atm
CO(g) + H2O(g) → H2(g) + CO2(g)
If a 10 L container initially has a partial pressure of 0.25 atm of CO and 1.35 atm of H2O at constant temperature of 1000 K. When the partial pressure of CO has dropped to 0.15 atm, what is the total pressure in the container?
A) 0.10 atm
B) 0.15 atm
C) 1.40 atm
D) 1.60 atm
E) 1.75 atm

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
16
If air has a concentration of CO2 of 380 ppm, what is the partial pressure of CO2 if the total pressure of 0.99 atm?
A) 3.76×10-4 atm
B) 3.84×10-4 atm
C) 3.84×10-3 atm
D) 3.76×10-1 atm
E) 3.84×10-1 atm
A) 3.76×10-4 atm
B) 3.84×10-4 atm
C) 3.84×10-3 atm
D) 3.76×10-1 atm
E) 3.84×10-1 atm

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
17
At room temperature which of the following gases will have the fastest average velocity?
A) H2
B) He
C) N2
D) O2
E) it depends on the pressure and volume
A) H2
B) He
C) N2
D) O2
E) it depends on the pressure and volume

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
18
From the kinetic theory of gases which do you predict is the fastest?
A) the most probable velocity of the gas.
B) the root mean square velocity of the gas.
C) the average velocity of the gas.
D) they will all be the same.
E) it depends on the molecular mass of the gas.
A) the most probable velocity of the gas.
B) the root mean square velocity of the gas.
C) the average velocity of the gas.
D) they will all be the same.
E) it depends on the molecular mass of the gas.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
19
Comparing the rms velocities H2 and He.
A) He is 2 times faster than H2
B) He is 2 times slower than H2
C) He is 4 times faster than H2
D) He is 4 times slower than H2
E) none of the above
A) He is 2 times faster than H2
B) He is 2 times slower than H2
C) He is 4 times faster than H2
D) He is 4 times slower than H2
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
20
What is the rms velocity of N2 gas at 100°C?
A) 18 m s-1
B) 36 m s-1
C) 156 m s1
D) 298 m s-1
E) 576 m s-1
A) 18 m s-1
B) 36 m s-1
C) 156 m s1
D) 298 m s-1
E) 576 m s-1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
21
The vibrational frequency of carbon monoxide is 6.42×1013 s-1. At 25°C what is the relative population of the first excited vibrational state compared to the ground state?
A) 3.2×10-5
B) 4.5×10-3
C) 0.62
D) 1.0
E) none of the above
A) 3.2×10-5
B) 4.5×10-3
C) 0.62
D) 1.0
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
22
The vibration frequency of HCl is 8.66×1013 s-1 and HBr is 7.68×1013 s-1. At 1000 K which molecules will have a larger population in the first excited vibrational state?
A) HCl
B) HBr
C) they will be exactly equal
D) it depends on the force constant of the bond
E) it depends on the total pressure
A) HCl
B) HBr
C) they will be exactly equal
D) it depends on the force constant of the bond
E) it depends on the total pressure

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
23
Comparing the van der Waals b constant for Xe with that of Ne
A) Xe will be larger
B) Ne will be larger
C) they will be exactly the same
D) either Xe or Ne will be larger depending on the temperature
E) either Xe or Ne will be larger depending on the pressure
A) Xe will be larger
B) Ne will be larger
C) they will be exactly the same
D) either Xe or Ne will be larger depending on the temperature
E) either Xe or Ne will be larger depending on the pressure

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
24
At the same temperature and pressure, comparing one mole of a gas that is described the following equation of state
P(V - nb) = nRT where b > 0
To one mole of an ideal gas
A) the volume will be larger than that of the ideal gas
B) the volume will be smaller than that of the ideal gas
C) the volume will be the same as that of the ideal gas
D) the volume will be larger or smaller depending on the temperature
E) the volume will be larger or smaller depending on the pressure
P(V - nb) = nRT where b > 0
To one mole of an ideal gas
A) the volume will be larger than that of the ideal gas
B) the volume will be smaller than that of the ideal gas
C) the volume will be the same as that of the ideal gas
D) the volume will be larger or smaller depending on the temperature
E) the volume will be larger or smaller depending on the pressure

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following in gases is least likely to behave ideally?
A) He
B) Ne
C) N2
D) H2
E) NH3
A) He
B) Ne
C) N2
D) H2
E) NH3

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
26
If the compressibility factor for a real gas yields a z < 1, then volume of that gas compared to the volume of an ideal gas at the same temperature and pressure would be
A) smaller volume than the ideal gas.
B) larger volume than the ideal gas.
C) identical volume to the ideal gas.
D) depend on the molecular weight of the gas.
E) depend on the temperature.
A) smaller volume than the ideal gas.
B) larger volume than the ideal gas.
C) identical volume to the ideal gas.
D) depend on the molecular weight of the gas.
E) depend on the temperature.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
27
Helium effuses through a small opening at a rate of 1×10-9 mol s-1. An unknown gas at the same temperature and pressure is found to effuse through the same opening at a rate of 3.78×10-10 mol s-1. What is the molecular mass of the unknown gas?
A) 4 g mol-1
B) 14 g mol-1
C) 18 g mol-1
D) 28 g mol-1
E) 40 g mol-1
A) 4 g mol-1
B) 14 g mol-1
C) 18 g mol-1
D) 28 g mol-1
E) 40 g mol-1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
28
Air is 78.1% N2 gas. This is the
A) percent by volume.
B) percent by mole.
C) percent by mass.
D) a & b
E) a & c
A) percent by volume.
B) percent by mole.
C) percent by mass.
D) a & b
E) a & c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
29
Which has more average kinetic energy per molecule, N2 gas at 25°C or He gas at 25°C?
A) N2
B) He
C) they are the same
D) it depends on the pressure
E) it depends on the volume
A) N2
B) He
C) they are the same
D) it depends on the pressure
E) it depends on the volume

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
30
A 5 L container is held at a constant temperature. Initially it has a partial pressure of 1.2 bar N2 and a partial pressure of 0.3 bars H2. The nitrogen and hydrogen react to form some NH3 and the total pressure is found to be 1.425 bar. What is the partial pressure of NH3?
A) 0.075 bar
B) 0.55 bar
C) 0.63 bar
D) 0.76 bar
E) 1.38 bar
A) 0.075 bar
B) 0.55 bar
C) 0.63 bar
D) 0.76 bar
E) 1.38 bar

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
31
Based on the van der Waals equation of state  Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas.
Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas. 
A) HCl
B) NO2
C) SO2
D) Must know n to answer this question
E) Must know P and V to answer this question
 Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas.
Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas. 
A) HCl
B) NO2
C) SO2
D) Must know n to answer this question
E) Must know P and V to answer this question

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
32
The compressibility factor (z) for an ideal gas is
A)

B) 0
C) 1
D) R
E) kB
A)

B) 0
C) 1
D) R
E) kB

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
33
With respect to the Maxwell-Boltzmann probability distribution function of molecular speeds, which of the following is true?
A)

B)

C)
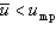
D)

E) A and D
A)

B)

C)
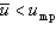
D)

E) A and D

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck


