Deck 6: Quantum Mechanics and Molecular Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/33
Play
Full screen (f)
Deck 6: Quantum Mechanics and Molecular Structure
1
In H2+, how many nodes does the 1su* orbital have along the internuclear axis?
A) 0
B) 0.5
C) 1
D) 2
E) 3
A) 0
B) 0.5
C) 1
D) 2
E) 3
C
2
If you excited the electron in H2+ from the 1sg state to the 1su* what would expect to happen to the molecule?
A) It would eject an electron to become two H+ ions
B) It would vibrate
C) It would contract to a shorter bond length
D) It would dissociate into an H atom and H+ ion
E) It would become a Helium atom
A) It would eject an electron to become two H+ ions
B) It would vibrate
C) It would contract to a shorter bond length
D) It would dissociate into an H atom and H+ ion
E) It would become a Helium atom
D
3
At large internuclear distances the 1pg orbital in H2+ resembles
A) the sum of two 1s hydrogen orbitals.
B) the difference of two 1s hydrogen orbitals.
C) the sum of two 2p hydrogen orbitals.
D) the difference of two 2p hydrogen orbitals.
E) the sum of a 1s and a 2p hydrogen orbital.
A) the sum of two 1s hydrogen orbitals.
B) the difference of two 1s hydrogen orbitals.
C) the sum of two 2p hydrogen orbitals.
D) the difference of two 2p hydrogen orbitals.
E) the sum of a 1s and a 2p hydrogen orbital.
D
4
Which of the following describe the 1su* orbital in H2+?
A) this is an antibonding orbital
B) this orbital has a node along the internuclear axis
C) this orbital is odd with respect to inversion
D) a & b
E) all of the above
A) this is an antibonding orbital
B) this orbital has a node along the internuclear axis
C) this orbital is odd with respect to inversion
D) a & b
E) all of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
5
Which will have a longer bond length, H2 or He2+?
A) H2
B) He2+
C) They will be exactly the same
D) There is no way to know
A) H2
B) He2+
C) They will be exactly the same
D) There is no way to know

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following has the highest bond order O2, O2+, N2, N2+?
A) O2
B) O2+
C) N2
D) N2+
E) N2+ and O2 are the same and the highest
A) O2
B) O2+
C) N2
D) N2+
E) N2+ and O2 are the same and the highest

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
7
What is the bond order in Cl2?
A) 0.5
B) 1
C) 1.5
D) 2
E) Cl2 won't form a bond
A) 0.5
B) 1
C) 1.5
D) 2
E) Cl2 won't form a bond

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
8
To be paramagnetic a molecule must have
A) an odd number of electrons.
B) an even number of electrons.
C) have at least one unpaired electron.
D) have at least two unpaired electrons.
E) b & d
A) an odd number of electrons.
B) an even number of electrons.
C) have at least one unpaired electron.
D) have at least two unpaired electrons.
E) b & d

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
9
If you remove one of the electrons from N2 what will happen to its bond length?
A) the bond length will increase
B) the bond length will decrease
C) the bond length will stay the same
D) there will no longer be a bond in the molecule
A) the bond length will increase
B) the bond length will decrease
C) the bond length will stay the same
D) there will no longer be a bond in the molecule

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
10
In the correlation diagram for HF the molecular orbitals are formed by mixing the 1s on the hydrogen with a 2p orbital in fluorine because
A) these orbitals are closest in energy
B) these orbitals have the same symmetry
C) these orbitals have the same angular momentum
D) a & b
E) all of the above
A) these orbitals are closest in energy
B) these orbitals have the same symmetry
C) these orbitals have the same angular momentum
D) a & b
E) all of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
11
In photoelectron spectroscopy if an electron is removed from a bonding orbital the vibrational fine structure will exhibit a
A) higher vibrational frequency than the parent molecule.
B) lower vibrational frequency than the parent molecule.
C) and identical vibrational frequency as the parent molecule.
D) there will be no vibrational fine structure in the spectrum.
A) higher vibrational frequency than the parent molecule.
B) lower vibrational frequency than the parent molecule.
C) and identical vibrational frequency as the parent molecule.
D) there will be no vibrational fine structure in the spectrum.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
12
The orbital energy of core electrons in a molecule are
A) nearly identical to the energy in the unbonded atom.
B) significantly higher than in the unbonded atom.
C) significantly lower than in the unbonded atom.
D) vary depending on the molecule.
E) vary depending on the atom.
A) nearly identical to the energy in the unbonded atom.
B) significantly higher than in the unbonded atom.
C) significantly lower than in the unbonded atom.
D) vary depending on the molecule.
E) vary depending on the atom.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
13
In a photoelectron spectrum of N2, you would expect the electrons coming off with the highest kinetic energy to be associated with which MO?
A) 1s N
B) sg2S
C) pu2p
D) s2pz
E) pg2p*
A) 1s N
B) sg2S
C) pu2p
D) s2pz
E) pg2p*

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
14
Why does the VB model predict there will be no bonding in BeH2?
A) There is no overlap between the atomic orbitals of Be and the H.
B) The orbitals of Be have the wrong symmetry compared to those in H.
C) The energy levels of the Be atomic orbitals are too high to mix with the H.
D) There are no unpaired electrons in the Be atomic orbitals.
E) The Be and H atomic orbitals have the same angular momentum.
A) There is no overlap between the atomic orbitals of Be and the H.
B) The orbitals of Be have the wrong symmetry compared to those in H.
C) The energy levels of the Be atomic orbitals are too high to mix with the H.
D) There are no unpaired electrons in the Be atomic orbitals.
E) The Be and H atomic orbitals have the same angular momentum.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
15
A simple VB wavefunction for Na2 would be
A) Yg = C1[1sA (1)1sB (2) + 1sA (2)1sB (1)]
B) Yg = C1[1sA (1)3sB (2) + 1sA (2)3sB (1)]
C) Yu = C1[3sA (1)3sB (2) - 3sA (2)3sB (1)]
D) Yg = C1[3sA (1)3sB (2) + 3sA (2)3sB (1)]
E) Yg = C1[1sA (1)3sB (2) - 3sA (2)3sB (1)]
A) Yg = C1[1sA (1)1sB (2) + 1sA (2)1sB (1)]
B) Yg = C1[1sA (1)3sB (2) + 1sA (2)3sB (1)]
C) Yu = C1[3sA (1)3sB (2) - 3sA (2)3sB (1)]
D) Yg = C1[3sA (1)3sB (2) + 3sA (2)3sB (1)]
E) Yg = C1[1sA (1)3sB (2) - 3sA (2)3sB (1)]

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
16
What would the VB model predict for the valency of C without "promotion" or "hybridization"?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
17
What are the VB wavefunctions for the bonds in NH3?
A) Ys = C1[1sH (1)1sN (2) + 1sH (2)1sN (1)]
B) Yp = C1[1sH (1)2pN (2) + 1sH (2)2pN (1)]
C) Ys = C1[1sH (1)2pN (2) - 1sH (2)2pN (1)]
D) Yp = C1[2pH (1)1sN (2) + 2pH (2)1sN (1)]
E) Ys = C1[1sH (1)2pN (2) + 1sH (2)2pN (1)]
A) Ys = C1[1sH (1)1sN (2) + 1sH (2)1sN (1)]
B) Yp = C1[1sH (1)2pN (2) + 1sH (2)2pN (1)]
C) Ys = C1[1sH (1)2pN (2) - 1sH (2)2pN (1)]
D) Yp = C1[2pH (1)1sN (2) + 2pH (2)1sN (1)]
E) Ys = C1[1sH (1)2pN (2) + 1sH (2)2pN (1)]

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
18
What do you predict for the hybridization of the central atom in CCl3?
A) not hybridized
B) sp3
C) sp2
D) sp
E) sp3d2
A) not hybridized
B) sp3
C) sp2
D) sp
E) sp3d2

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
19
In the molecule, H-CºN, the VB model would describe the sigma bond between the C and the N as a combination of
A) 2p orbital on the C and a 2p orbital on the N.
B) 2p orbital on the C and a 2s orbital on the N.
C) 2s orbital on the C and a 2p orbital on the N.
D) a sp hybridized orbital on the C and a 2p orbital on the N.
E) a sp3 hybridized orbital on the C and a 2p orbital on the N.
A) 2p orbital on the C and a 2p orbital on the N.
B) 2p orbital on the C and a 2s orbital on the N.
C) 2s orbital on the C and a 2p orbital on the N.
D) a sp hybridized orbital on the C and a 2p orbital on the N.
E) a sp3 hybridized orbital on the C and a 2p orbital on the N.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
20
In the molecule, H-CºN, the VB model would predict how many s and p bonds?
A) 1s and 2p
B) 2s and 2p
C) 2s and 1p
D) 1s and 1p
E) 1s and 3p
A) 1s and 2p
B) 2s and 2p
C) 2s and 1p
D) 1s and 1p
E) 1s and 3p

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
21
In the LCAO wavefunction for H2 given below, the second term can be described as "ionic" because ![<strong>In the LCAO wavefunction for H<sub>2</sub> given below, the second term can be described as ionic because = [1s<sup>A</sup> (1)1s<sup>B</sup> (2) + 1s<sup>A</sup> (2)1s<sup>B</sup> (1)] + [1s<sup>A</sup> (1)1s<sup>A</sup> (2) + 1s<sup>B</sup> (1)1s<sup>B</sup> (2)]</strong> A) it describes the excited state of each orbital. B) it has both electrons centered on one nucleus or the other. C) it has both electrons equally shared between the two nuclei. D) it is a wavefunction that describes two electrons. E) the second term is not an ionic term.](https://storage.examlex.com/TB10703/11ed398b_8361_6967_a786_61d992820aba_TB10703_11.jpg) = [1sA (1)1sB (2) + 1sA (2)1sB (1)] + [1sA (1)1sA (2) + 1sB (1)1sB (2)]
= [1sA (1)1sB (2) + 1sA (2)1sB (1)] + [1sA (1)1sA (2) + 1sB (1)1sB (2)]
A) it describes the excited state of each orbital.
B) it has both electrons centered on one nucleus or the other.
C) it has both electrons equally shared between the two nuclei.
D) it is a wavefunction that describes two electrons.
E) the second term is not an "ionic" term.
![<strong>In the LCAO wavefunction for H<sub>2</sub> given below, the second term can be described as ionic because = [1s<sup>A</sup> (1)1s<sup>B</sup> (2) + 1s<sup>A</sup> (2)1s<sup>B</sup> (1)] + [1s<sup>A</sup> (1)1s<sup>A</sup> (2) + 1s<sup>B</sup> (1)1s<sup>B</sup> (2)]</strong> A) it describes the excited state of each orbital. B) it has both electrons centered on one nucleus or the other. C) it has both electrons equally shared between the two nuclei. D) it is a wavefunction that describes two electrons. E) the second term is not an ionic term.](https://storage.examlex.com/TB10703/11ed398b_8361_6967_a786_61d992820aba_TB10703_11.jpg) = [1sA (1)1sB (2) + 1sA (2)1sB (1)] + [1sA (1)1sA (2) + 1sB (1)1sB (2)]
= [1sA (1)1sB (2) + 1sA (2)1sB (1)] + [1sA (1)1sA (2) + 1sB (1)1sB (2)]A) it describes the excited state of each orbital.
B) it has both electrons centered on one nucleus or the other.
C) it has both electrons equally shared between the two nuclei.
D) it is a wavefunction that describes two electrons.
E) the second term is not an "ionic" term.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
22
A Lewis dot structure for benzene, C6H6 would give two equivalent resonance structures for this cyclic molecule with alternating single and double bonds  A combined LCAO and MO picture for the bonding of the carbon atoms in benzene would be
A combined LCAO and MO picture for the bonding of the carbon atoms in benzene would be
A) 3s(C2p-C2p) bonds between each C atom
B) 2s(Csp3-Csp3) bonds between each C atom, and 1p MO delocalized over the C's
C) 1s(Csp3-Csp3) bonds between each C atom, and 1p MO delocalized over the C's
D) 1s(Csp2-Csp2) bonds between each C atom, and 2p MO delocalized over the C's
E) 1s(Csp2-Csp2) bonds between each C atom, and 3p MO delocalized over the C's
 A combined LCAO and MO picture for the bonding of the carbon atoms in benzene would be
A combined LCAO and MO picture for the bonding of the carbon atoms in benzene would beA) 3s(C2p-C2p) bonds between each C atom
B) 2s(Csp3-Csp3) bonds between each C atom, and 1p MO delocalized over the C's
C) 1s(Csp3-Csp3) bonds between each C atom, and 1p MO delocalized over the C's
D) 1s(Csp2-Csp2) bonds between each C atom, and 2p MO delocalized over the C's
E) 1s(Csp2-Csp2) bonds between each C atom, and 3p MO delocalized over the C's

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
23
VB theory is poorly suited to polar molecules such as HCl because
A) VB theory is a localized bonding model
B) VB theory assumes bonds form only between unpaired electrons in atomic orbitals
C) VB theory can't predict p bonds
D) VB wavefunctions always have even parity with respect to inversion
E) VB wavefunctions do not contain any ionic states
A) VB theory is a localized bonding model
B) VB theory assumes bonds form only between unpaired electrons in atomic orbitals
C) VB theory can't predict p bonds
D) VB wavefunctions always have even parity with respect to inversion
E) VB wavefunctions do not contain any ionic states

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
24
The Born Openheimer approximation assumes that the electrons move much faster than the nuclei. This lets us assume the wavefunctions for the electrons in the molecule
A) are independent of the position of the nuclei.
B) depend only on the positions of the nuclei.
C) are the same as the wavefunctions for the nuclei.
D) the product of one electron wavefunctions.
E) always even with regard to inversion symmetry.
A) are independent of the position of the nuclei.
B) depend only on the positions of the nuclei.
C) are the same as the wavefunctions for the nuclei.
D) the product of one electron wavefunctions.
E) always even with regard to inversion symmetry.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
25
The lowest energy antibonding orbital in H2+
A) has a node along the internuclear axis
B) has an energy that is higher than H + H+
C) is antisymmetric with respect to inversion
D) a & b
E) all of the above
A) has a node along the internuclear axis
B) has an energy that is higher than H + H+
C) is antisymmetric with respect to inversion
D) a & b
E) all of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
26
The ionization energy of H2- would be expected to be
A) higher than that of H2
B) higher than that of H
C) lower than that of H2
D) lower than that of H
E) a & b
F) c & d
A) higher than that of H2
B) higher than that of H
C) lower than that of H2
D) lower than that of H
E) a & b
F) c & d

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
27
A MO orbital for a heteronuclear diatomic is given by 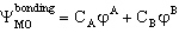 If A is more electronegative than B then you would predict that
If A is more electronegative than B then you would predict that
A) CA > CB
B) CA = CB
C) CA < CB
D) CA = -CB
E) you would not expect any relation between these coefficients and the electronegativity of the elements A & B
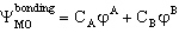 If A is more electronegative than B then you would predict that
If A is more electronegative than B then you would predict thatA) CA > CB
B) CA = CB
C) CA < CB
D) CA = -CB
E) you would not expect any relation between these coefficients and the electronegativity of the elements A & B

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
28
The molecule HeH+ has an antibonding MO of the form  For this you would expect
For this you would expect
A) C1 > C2
B) C2 < C2
C) C1 = C2
 For this you would expect
For this you would expectA) C1 > C2
B) C2 < C2
C) C1 = C2

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
29
VB theory and MO theory both predict that N2 has a bond order of three, one sigma bond and two pi bonds. What is the difference between these two models?
A) The wavefunctions for the electrons are different
B) The energies predicted would be different
C) both a & b
D) There are no differences between VB and MO for N2
A) The wavefunctions for the electrons are different
B) The energies predicted would be different
C) both a & b
D) There are no differences between VB and MO for N2

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
30
In CO2 the hybridization of the C atom is
A) not hybridized
B) sp
C) sp2
D) sp3
E) sp3d2
A) not hybridized
B) sp
C) sp2
D) sp3
E) sp3d2

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
31
Of the following homonuclear diatomic molecules, which is paramagnetic?
A) B2
B) C2
C) N2
D) F2
E) None of the above
A) B2
B) C2
C) N2
D) F2
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
32
Oxygen is paramagnetic and has a bond order of two. Which of the following represents the ground electronic state for oxygen?
A)
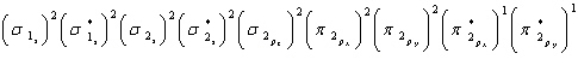
B)

C)
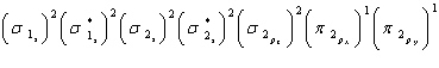
D) A and C are equally probable
E) None of the above
A)
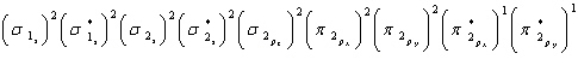
B)

C)
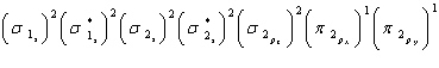
D) A and C are equally probable
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following combinations of atomic orbitals will result in a bonding molecular orbital for a homonuclear diatomic molecule when the z-axis is aligned with the nuclei of the bonded atom?
A)

B)
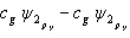
C)
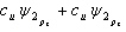
D)

E) A, B, and D
A)

B)
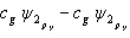
C)
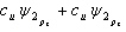
D)

E) A, B, and D

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck


