Deck 4: Introduction to Quantum Mechanics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/33
Play
Full screen (f)
Deck 4: Introduction to Quantum Mechanics
1
Electromagnetic radiation cannot typically pass through conductive metal mesh in which the holes are smaller than the wavelength of the radiation. Which is the largest mesh that would effectively block out radiation at 5 GHz?
A) 1 m
B) 10 cm
C) 1 mm
D) 100 mm
E) 10 nm
A) 1 m
B) 10 cm
C) 1 mm
D) 100 mm
E) 10 nm
C
2
Femtosecond lasers can produce very short pulses of light. How long would it take a short pulse of light to travel across a 3 m table?
A) 10-15 s
B) 10-12 s
C) 10-8 s
D) 10-4 s
E) 10 s
A) 10-15 s
B) 10-12 s
C) 10-8 s
D) 10-4 s
E) 10 s
C
3
What is the frequency of x-ray radiation with a wavelength of 1 nm?
A) 1×10-9 s-1
B) 3×10-9 s-1
C) 1×109 s-1
D) 3×1015 s-1
E) 3×1017 s-1
A) 1×10-9 s-1
B) 3×10-9 s-1
C) 1×109 s-1
D) 3×1015 s-1
E) 3×1017 s-1
E
4
The index of refraction is the ratio of the speed of light in that medium to the speed of light in vacuum. If it takes a pulse of 500 nm light 0.15 ns to travel through a 3 cm block of glass, what is the index of refraction of the glass?
A) 0.0015
B) 1.5
C) 10
D) 30
E) 500
A) 0.0015
B) 1.5
C) 10
D) 30
E) 500

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
5
Increasing the temperature of a black-body will cause
A) the total intensity of emitted radiation to increase
B) the peak of the emission spectrum to shift to longer wavelengths
C) the peak of the emission spectrum to shift to shorter wavelengths
D) both a & b
E) both a & c
A) the total intensity of emitted radiation to increase
B) the peak of the emission spectrum to shift to longer wavelengths
C) the peak of the emission spectrum to shift to shorter wavelengths
D) both a & b
E) both a & c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
6
A laser emits 10 mJ pulses of 532 nm light. How many photons are in each pulse?
A) 2.68×1016
B) 3.73×1017
C) 2.52×1018
D) 3.73×1018
E) 5.36×1018
A) 2.68×1016
B) 3.73×1017
C) 2.52×1018
D) 3.73×1018
E) 5.36×1018

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
7
Hydrogen has a strong emission line at 6563 Å. What is the energy difference between the energy levels in hydrogen that are involved in this transition?
A) 6.56×10-10 J
B) 4.67×10-14 J
C) 1.07×10-19 J
D) 3.03×10-19 J
E) 6.56×10-19 J
A) 6.56×10-10 J
B) 4.67×10-14 J
C) 1.07×10-19 J
D) 3.03×10-19 J
E) 6.56×10-19 J

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
8
In the Franck-Hertz experiment on potassium shows a threshold occurs at 1.55 eV, what which of the following wavelengths would you expect to observe just above this threshold?
A) 155 nm
B) 374 nm
C) 400 nm
D) 600 nm
E) 800 nm
A) 155 nm
B) 374 nm
C) 400 nm
D) 600 nm
E) 800 nm

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
9
Bohr in his model of the atom assumed that what was quantized?
A) the radius of the electron orbit
B) the energy of the electron
C) the kinetic energy of the electron
D) the angular momentum of the electron
E) the coulomb potential
A) the radius of the electron orbit
B) the energy of the electron
C) the kinetic energy of the electron
D) the angular momentum of the electron
E) the coulomb potential

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
10
According to Bohr's model of the atom, which is the largest radius?
A) the n=1 state of H
B) the n=2 state of H
C) the n=3 state of Li2+
D) the n=3 state of H
E) the n=4 state of He+
A) the n=1 state of H
B) the n=2 state of H
C) the n=3 state of Li2+
D) the n=3 state of H
E) the n=4 state of He+

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
11
In the photoelectric effect, increasing the intensity of the light will
A) ensure that electrons will be emitted from all metals.
B) cause more electrons to be emitted from the metal if the frequency is sufficiently high.
C) cause the electrons to be emitted with higher kinetic energy if the frequency is sufficiently high.
D) have no effect on the experiment.
E) both b and c
A) ensure that electrons will be emitted from all metals.
B) cause more electrons to be emitted from the metal if the frequency is sufficiently high.
C) cause the electrons to be emitted with higher kinetic energy if the frequency is sufficiently high.
D) have no effect on the experiment.
E) both b and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
12
In the photoelectric effect, increasing the frequency of the light
A) cause fewer electrons to be emitted from the metal if the frequency is sufficiently high.
B) cause more electrons to be emitted from the metal if the frequency is sufficiently high.
C) cause the electrons to be emitted with higher kinetic energy if the frequency is sufficiently high.
D) have no effect on the experiment.
E) both b and c
A) cause fewer electrons to be emitted from the metal if the frequency is sufficiently high.
B) cause more electrons to be emitted from the metal if the frequency is sufficiently high.
C) cause the electrons to be emitted with higher kinetic energy if the frequency is sufficiently high.
D) have no effect on the experiment.
E) both b and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
13
Estimate the de Broglie wavelength for a golf ball traveling at 10 m s-1
A) 10-51 m
B) 10-35 m
C) 10-21 m
D) 10-6 m
E) 10 m
A) 10-51 m
B) 10-35 m
C) 10-21 m
D) 10-6 m
E) 10 m

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
14
What is the de Broglie wavelength of an electron traveling at 1×105 m s-1
A) 6.6×10-39 m
B) 6.6×10-30 m
C) 4.2×10-18 m
D) 7.3×10-9 m
E) 5.1×10-8 m
A) 6.6×10-39 m
B) 6.6×10-30 m
C) 4.2×10-18 m
D) 7.3×10-9 m
E) 5.1×10-8 m

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
15
Which has a shorter wavelength: an electron with a kinetic energy of 4×10-19 J or a photon with an energy of 4×10-19 J?
A) the electron
B) the photon
C) they will have the same wavelength
D) there is no way to know
A) the electron
B) the photon
C) they will have the same wavelength
D) there is no way to know

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
16
Calcium has a work function of 4.64×10-19 J. Which of the following wavelengths will eject electrons from calcium?
A) 375.2 nm
B) 401.8 nm
C) 442.4 nm
D) a & b
E) a, b, & c
A) 375.2 nm
B) 401.8 nm
C) 442.4 nm
D) a & b
E) a, b, & c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
17
The position of an electron in a Be2+ ion is known to 5.0×10-11 m. What is the uncertainty in its velocity?
A) 2.0×105 m s-1
B) 1.2×106 m s-1
C) 4.6×106 m s-1
D) 9.2×106 m s-1
E) 3.0×108 m s-1
A) 2.0×105 m s-1
B) 1.2×106 m s-1
C) 4.6×106 m s-1
D) 9.2×106 m s-1
E) 3.0×108 m s-1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
18
The uncertainty in the velocity of an electron is 1×105 m s-1. What is the minimum uncertainty in its position?
A) 5.79×10-5 m
B) 2.31×10-9 m
C) 5.79×10-10 m
D) 2.31×10-10 m
E) 1.08×10-10
A) 5.79×10-5 m
B) 2.31×10-9 m
C) 5.79×10-10 m
D) 2.31×10-10 m
E) 1.08×10-10

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
19
The wave function for a n=5 state of the particle in a box has how many nodes?
A) 1
B) 3
C) 4
D) 5
E) 6
A) 1
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
20
For a particle in a cubic box, how many energy levels have an energy that is 2 times the energy of the ground state?
A) 0
B) 1
C) 2
D) 3
E) 8
A) 0
B) 1
C) 2
D) 3
E) 8

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
21
For the a particle in a box in the n = 1 state, you are most likely to find the particle
A) at the left edge of the box.
B) at the right edge of the box.
C) in the middle of the box.
D) the particle is equally likely to be found at all positions.
E) it depends on the energy.
A) at the left edge of the box.
B) at the right edge of the box.
C) in the middle of the box.
D) the particle is equally likely to be found at all positions.
E) it depends on the energy.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
22
For the one dimensional particle in a box if you double the length of the box the ground state energy will
A) decrease by a factor of 4.
B) decrease by a factor of 2.
C) stay the same.
D) increase by a factor of 2.
E) increase by a factor of 4.
A) decrease by a factor of 4.
B) decrease by a factor of 2.
C) stay the same.
D) increase by a factor of 2.
E) increase by a factor of 4.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
23
If the vibrational frequency of H35Cl is 8.66×1013 Hz, what do you think the vibrational frequency of H37Cl would be?
A) 4.33×1013 Hz
B) 8.20×1013 Hz
C) 8.66×1013 Hz
D) 9.15×1013 Hz
E) 1.73×1014 Hz
A) 4.33×1013 Hz
B) 8.20×1013 Hz
C) 8.66×1013 Hz
D) 9.15×1013 Hz
E) 1.73×1014 Hz

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
24
If the bond in carbon monoxide is modeled as a harmonic oscillator the force constant is 1860 N m-1, and the reduced mass is 6.86 amu. What is the energy of the ground vibrational state of CO?
A) 1.35×10-21 J
B) 2.71×10-21 J
C) 3.53×10-21 J
D) 2.13×10-20 J
E) 4.26×10-20 J
A) 1.35×10-21 J
B) 2.71×10-21 J
C) 3.53×10-21 J
D) 2.13×10-20 J
E) 4.26×10-20 J

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
25
In Bohr's model of the atom the radius of an electron in the n=2 orbit is
A) 0.25 times the radius of the n=1 orbit
B) 0.5 times the radius of the n=1 orbit
C) the same as the radius of the n=1 orbit
D) 2 times the radius of the n=1 orbit
E) 4 times the radius of the n=1 orbit
A) 0.25 times the radius of the n=1 orbit
B) 0.5 times the radius of the n=1 orbit
C) the same as the radius of the n=1 orbit
D) 2 times the radius of the n=1 orbit
E) 4 times the radius of the n=1 orbit

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
26
What is the probability of finding a particle in a box of length L between zero and L/2?
A) 1
B) 0.5
C) 0.25
D) L/2
E) it depends on the quantum number n
A) 1
B) 0.5
C) 0.25
D) L/2
E) it depends on the quantum number n

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
27
The work function for aluminum is 6.53×10-19 J. If a surface of aluminum is irradiated with 400 nm what is the maximum kinetic energy of the electrons emitted from the surface due to the photo-electric effect?
A) 4.13×105 m s-1
B) 5.85×105 m s-1
C) 1.04×106 m s-1
D) 1.20×106 m s-1
E) no electrons will be emitted
A) 4.13×105 m s-1
B) 5.85×105 m s-1
C) 1.04×106 m s-1
D) 1.20×106 m s-1
E) no electrons will be emitted

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
28
Which has the shortest wavelength?
A) 500 nm light
B) 1015 Hz light
C) an electron traveling at 2×105 m s-1
D) a 10 g mass traveling at 10 m s-1
E) there is no way to know
A) 500 nm light
B) 1015 Hz light
C) an electron traveling at 2×105 m s-1
D) a 10 g mass traveling at 10 m s-1
E) there is no way to know

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
29
According to the Bohr model of the atom, which is highest in energy?
A) n=1 electron in He+
B) n=2 electron in H
C) n=2 electron in He+
D) n=3 electron in Li2+
E) n=3 electron in H
A) n=1 electron in He+
B) n=2 electron in H
C) n=2 electron in He+
D) n=3 electron in Li2+
E) n=3 electron in H

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
30
Assuming they have the same force constant, the ratio the vibrational frequency for 16O2 to 18O2 is
A) 8 to 9
B) 9 to 8
C) 3 to Ö8
D) Ö8 to 3
E) they are the same
A) 8 to 9
B) 9 to 8
C) 3 to Ö8
D) Ö8 to 3
E) they are the same

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
31
The energy of electromagnetic radiation is directly proportional to...
A) The amplitude of the wave
B) The frequency of the wave
C) The wavelength of the wave
D) A and B
E) B and C
A) The amplitude of the wave
B) The frequency of the wave
C) The wavelength of the wave
D) A and B
E) B and C

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
32
Radiation from which region of the electromagnetic spectrum will induce molecular vibrations without inducing electronic transitions?
A) ultraviolet
B) X-rays
C) microwave
D) infrared
E)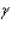 -rays
-rays
A) ultraviolet
B) X-rays
C) microwave
D) infrared
E)
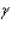 -rays
-rays
Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
33
Proper wavefunctions (  ) in quantum mechanics must be normalized. Which statement below best summarizes this mathematically?
) in quantum mechanics must be normalized. Which statement below best summarizes this mathematically?
A)
B)
C)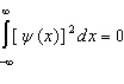
D)

E)
 ) in quantum mechanics must be normalized. Which statement below best summarizes this mathematically?
) in quantum mechanics must be normalized. Which statement below best summarizes this mathematically?A)

B)

C)
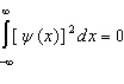
D)


E)


Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck


