Deck 3: Chemical Bonding: the Classical Description
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/33
Play
Full screen (f)
Deck 3: Chemical Bonding: the Classical Description
1
Before the discovery of the element germanium in 1886, its existence was predicted by Mendeleev as "eka-silicon". Mendeleev not only predicted the existence of this element but also its properties. Use the density of the neighboring elements to estimate the density of Ge. 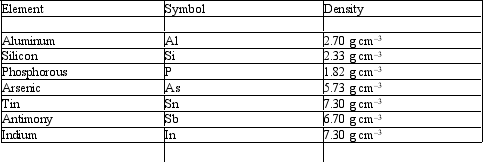
A) 2.5 g cm-3
B) 4 g cm-3
C) 5.5 g cm-3
D) 7 g cm-3
E) 9.6 g cm-3

A) 2.5 g cm-3
B) 4 g cm-3
C) 5.5 g cm-3
D) 7 g cm-3
E) 9.6 g cm-3
C
2
The Group II alkaline earth metals for complexes with the Group VII Halogens with a ratio for Group II element: Group VII element that is
A) 1:1
B) 2:1
C) 1:2
D) 2:7
E) 7:2
A) 1:1
B) 2:1
C) 1:2
D) 2:7
E) 7:2
C
3
The elements in Group VI, the chalcogens, make compounds with hydrogen that have the generic formula.
A) RH
B) RH2
C) RH3
D) RH4
E) are not known to form compounds with hydrogen
A) RH
B) RH2
C) RH3
D) RH4
E) are not known to form compounds with hydrogen
B
4
Which of the following has the lowest potential energy?
A) an electron and a hydrogen nucleus separated by 4Å
B) an electron and a helium nucleus separated by 4Å
C) an electron and a hydrogen nucleus separated by 2Å
D) an electron and a lithium nucleus separated by 4Å
E) an electron and a gold nucleus separated by 10Å
A) an electron and a hydrogen nucleus separated by 4Å
B) an electron and a helium nucleus separated by 4Å
C) an electron and a hydrogen nucleus separated by 2Å
D) an electron and a lithium nucleus separated by 4Å
E) an electron and a gold nucleus separated by 10Å

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
5
Which is the largest energy?
A) The first ionization energy of O
B) The first ionization energy of F
C) The first ionization energy of Ne
D) The first ionization energy of Na
E) The second ionization energy of Na
A) The first ionization energy of O
B) The first ionization energy of F
C) The first ionization energy of Ne
D) The first ionization energy of Na
E) The second ionization energy of Na

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
6
Below is a graph of the ionization energies for a particular element. 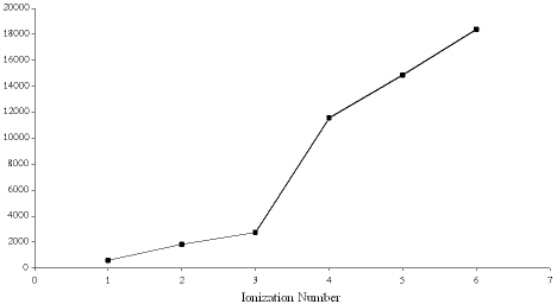 Based on the trend in the ionization energies the element is most likely
Based on the trend in the ionization energies the element is most likely
A) Ne
B) B
C) S
D) Al
E) K
 Based on the trend in the ionization energies the element is most likely
Based on the trend in the ionization energies the element is most likelyA) Ne
B) B
C) S
D) Al
E) K

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
7
Arrange the following in order of increasing electronegativity: C, F, Na, O
A) C, O, F, Na
B) Na, O, C, F
C) Na, C, F, O
D) Na, C, O, F
E) F, O, Ca, Na
A) C, O, F, Na
B) Na, O, C, F
C) Na, C, F, O
D) Na, C, O, F
E) F, O, Ca, Na

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements best describes electronegativity trends moving from left to right across the periodic table?
A) always decreases
B) mostly decreases
C) always increases
D) mostly increases
E) shows no trend across the periodic table only up and down
A) always decreases
B) mostly decreases
C) always increases
D) mostly increases
E) shows no trend across the periodic table only up and down

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
9
Ba2+ has
A) 0 valence electrons and 54 core electrons
B) 0 valence electrons and 56 core electrons
C) 2 valence electrons and 52 core electrons
D) 2 valence electrons and 54 core electrons
E) 2 valence electrons and 56 core electrons
A) 0 valence electrons and 54 core electrons
B) 0 valence electrons and 56 core electrons
C) 2 valence electrons and 52 core electrons
D) 2 valence electrons and 54 core electrons
E) 2 valence electrons and 56 core electrons

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
10
Given the following the data, what is the energy change for this reaction?  Na(g) + Cl(g) → Na+(g) + Cl-(g)
Na(g) + Cl(g) → Na+(g) + Cl-(g)
A) -1198.4 kJ mol-1
B) -146.8 kJ mol-1
C) +146.8 kJ mol-1
D) +672.6 kJ mol-1
E) +1198.4 kJ mol-1
 Na(g) + Cl(g) → Na+(g) + Cl-(g)
Na(g) + Cl(g) → Na+(g) + Cl-(g)A) -1198.4 kJ mol-1
B) -146.8 kJ mol-1
C) +146.8 kJ mol-1
D) +672.6 kJ mol-1
E) +1198.4 kJ mol-1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
11
Based on Coulombic forces, which would you expect to have the strongest ionic bond?
A) NaCl
B) KCl
C) NaBr
D) MgF2
E) MgO
A) NaCl
B) KCl
C) NaBr
D) MgF2
E) MgO

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
12
Use electronegativity to rank the following in order of increasing polarity: FCl, Cl2, BrCl, ICl
A) FCl, Cl2, BrCl, ICl
B) ICl, BrCl, Cl2, FCl
C) Cl2, FCl, BrCl, ICl
D) Cl2, ICl, BrCl, FCl
E) Cl2, BrCl, ICl, FCl
A) FCl, Cl2, BrCl, ICl
B) ICl, BrCl, Cl2, FCl
C) Cl2, FCl, BrCl, ICl
D) Cl2, ICl, BrCl, FCl
E) Cl2, BrCl, ICl, FCl

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
13
A HCl molecule has a bond length of 1.284 Å and a dipole moment of 1.109 Debye. What is the percent ionic character of the HCl bond?
A) 10%
B) 18%
C) 54%
D) 92%
E) 100%
A) 10%
B) 18%
C) 54%
D) 92%
E) 100%

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
14
A KF molecule has a dipole moment of 8.59 Debye and has a bond that can be described as having 82% ionic character. If the bond were 100% ionic what would the dipole moment be?
A) 7.04 D
B) 8.59 D
C) 10.14 D
D) 10.76 D
E) 26.59 D
A) 7.04 D
B) 8.59 D
C) 10.14 D
D) 10.76 D
E) 26.59 D

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
15
Use electronegativity difference to arrange the following bonds in order of increasing polarity:
C-H, N-H, O-H, F-H
A) C-H, N-H, O-H, F-H
B) C-H, O-H, N-H, F-H
C) O-H, N-H, C-H, F-H
D) F-H, N-H, O-H, C-H
E) F-H, O-H, N-H, C-H
C-H, N-H, O-H, F-H
A) C-H, N-H, O-H, F-H
B) C-H, O-H, N-H, F-H
C) O-H, N-H, C-H, F-H
D) F-H, N-H, O-H, C-H
E) F-H, O-H, N-H, C-H

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
16
HCl has a bond length of 1.284 Å and a bond energy of 429 kJ mol-1. HI has a bond length of 1.620 Å and a bond energy of 295 kJ mol-1. Based on this, one might expect that HBr would have the following bond length and bond energy
A) 1.8 Å and 200 kJ mol-1
B) 1.4 Å and 360 kJ mol-1
C) 1.2 Å and 500 kJ mol-1
D) 1.3 Å and 430 kJ mol-1
E) it should not follow any trend
A) 1.8 Å and 200 kJ mol-1
B) 1.4 Å and 360 kJ mol-1
C) 1.2 Å and 500 kJ mol-1
D) 1.3 Å and 430 kJ mol-1
E) it should not follow any trend

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is the best Lewis diagram for formaldehyde, CH2O?
A)
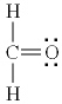
B)
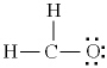
C)
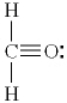
D)
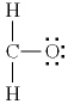
E)
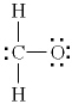
A)

B)
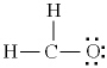
C)

D)
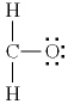
E)


Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
18
Choose the best statement regarding why diagram I is the better choice between these two Lewis diagrams for N2O 
A) because II does not satisfy the octet rule for all atoms.
B) because structure I has fewer formal charges than II.
C) because structure I has the negative formal charge on the most electronegative atom.
D) because structure I has the positive formal charge on the most electronegative atom.
E) the best structure is actually structure II.

A) because II does not satisfy the octet rule for all atoms.
B) because structure I has fewer formal charges than II.
C) because structure I has the negative formal charge on the most electronegative atom.
D) because structure I has the positive formal charge on the most electronegative atom.
E) the best structure is actually structure II.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
19
Draw a Lewis diagram for HCN to answer the following about the bond order of the C-N bond and the lone pairs on the nitrogen.
A) single bond, three lone pairs
B) double bond, two lone pairs
C) double bond, one lone pair
D) triple bond, one lone pair
E) triple bond, zero lone pair
A) single bond, three lone pairs
B) double bond, two lone pairs
C) double bond, one lone pair
D) triple bond, one lone pair
E) triple bond, zero lone pair

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
20
In the Lewis diagram for SCN-, the formal charges are 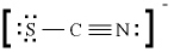
A) S (-1), C (0), N (+1)
B) S (-1), C (0), N (0)
C) S (0), C (-1), N (0)
D) S (-1), C (+1), N (-1)
E) S (+1), C (0), N (-1)
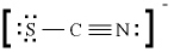
A) S (-1), C (0), N (+1)
B) S (-1), C (0), N (0)
C) S (0), C (-1), N (0)
D) S (-1), C (+1), N (-1)
E) S (+1), C (0), N (-1)

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
21
Below are two equivalent resonance structures for ozone, O3
 Which of the following would you expect for molecular ozone?
Which of the following would you expect for molecular ozone?
A) it has one O-O bond that is shorter than the other.
B) it has one O-O bond that alternates between being shorter and longer than the other.
C) it has two O-O bonds that are identical.
D) the partial charge on the oxygen atoms on each end of the molecule are different.
E) both b & d
 Which of the following would you expect for molecular ozone?
Which of the following would you expect for molecular ozone?A) it has one O-O bond that is shorter than the other.
B) it has one O-O bond that alternates between being shorter and longer than the other.
C) it has two O-O bonds that are identical.
D) the partial charge on the oxygen atoms on each end of the molecule are different.
E) both b & d

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
22
When drawing the Lewis diagram phosphine, PH3, how many equivalent resonance structures provide can be drawn that provide a Nobel gas configuration for each atom?
A) there are four equivalent resonance structures
B) there are three equivalent resonance structures
C) there are two equivalent resonance structures
D) there is only one structure
E) there are no structures that provide a Nobel gas configuration for each atom
A) there are four equivalent resonance structures
B) there are three equivalent resonance structures
C) there are two equivalent resonance structures
D) there is only one structure
E) there are no structures that provide a Nobel gas configuration for each atom

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
23
Using VSEPR theory, give the steric number and name the geometry for the molecule, AsF3
A) steric number 4, tetrahedral
B) steric number 3, pyramidal
C) steric number 4, pyramidal
D) steric number 3, bent
E) steric number 3, linear
A) steric number 4, tetrahedral
B) steric number 3, pyramidal
C) steric number 4, pyramidal
D) steric number 3, bent
E) steric number 3, linear

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
24
Using VSEPR theory including any distortions from the lone pairs estimate the S-C-S angle in the molecule CS2
A) 107°
B) 109.5°
C) 111°
D) 178°
E) 180°
A) 107°
B) 109.5°
C) 111°
D) 178°
E) 180°

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
25
Which will have the smallest angle between the hydrogens and the central atom?
A) CH4
B) NH4+
C) NH3
D) OH2
E) they will all be exactly the same
A) CH4
B) NH4+
C) NH3
D) OH2
E) they will all be exactly the same

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
26
What geometry would you expect for the nitrate ion, NO3- based on VSEPR theory?
A) linear
B) bent
C) trigonal planar
D) pyramidal
E) distorted T
A) linear
B) bent
C) trigonal planar
D) pyramidal
E) distorted T

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
27
In the dichromate ion Cr2O72- the chromium atom has what oxidation number?
A) -1
B) +3
C) +4
D) +6
E) +7
A) -1
B) +3
C) +4
D) +6
E) +7

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
28
In the compound magnesium hydride, MgH2, the hydrogen has an oxidation number of
A) +2
B) +1
C) 0
D) -1
E) -2
A) +2
B) +1
C) 0
D) -1
E) -2

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
29
The molecular formula for iron(III)oxide is?
A) FeO3
B) Fe2O3
C) Fe3O2
D) Fe3O
E) Fe3O3
A) FeO3
B) Fe2O3
C) Fe3O2
D) Fe3O
E) Fe3O3

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
30
Provide a name for the compound Ca(ClO3)2.
A) calcium chlorate
B) calcium chlorite
C) calcium dichloride
D) calcium chloride
E) calcium chlorine
A) calcium chlorate
B) calcium chlorite
C) calcium dichloride
D) calcium chloride
E) calcium chlorine

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
31
Using VESPR theory, give the geometry of the molecule TeCl4.
A) Tetrahedral
B) Square planar
C) See-saw
D) Square pyramidal
E) None of the above
A) Tetrahedral
B) Square planar
C) See-saw
D) Square pyramidal
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
32
Which species must be polar?
A) C2H4
B) CH4
C) CO2
D) CO
E) None of the above
A) C2H4
B) CH4
C) CO2
D) CO
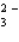
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
33
A quantum chemistry program indicates that the dipole moment of PH3 is zero. Which statement(s) best explains this result?
A) PH3 is a trigonal planar structure and the bond dipoles cancel.
B) PH3 violates the octet rule and does not exist.
C) The electronegativities of P and H are very close in value; therefore, no bond dipoles exist.
D) A and C only
E) A, B, and C
A) PH3 is a trigonal planar structure and the bond dipoles cancel.
B) PH3 violates the octet rule and does not exist.
C) The electronegativities of P and H are very close in value; therefore, no bond dipoles exist.
D) A and C only
E) A, B, and C

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck


