Deck 1: The Atom in Modern Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/33
Play
Full screen (f)
Deck 1: The Atom in Modern Chemistry
1
Air is best classified as
A) an element
B) a compound
C) a homogeneous mixture
D) a heterogeneous mixture
A) an element
B) a compound
C) a homogeneous mixture
D) a heterogeneous mixture
C
2
A marble tabletop is best classified as
A) an element
B) a compound
C) a homogeneous mixture
D) a heterogeneous mixture
A) an element
B) a compound
C) a homogeneous mixture
D) a heterogeneous mixture
D
3
A pure substance that is composed of only one type of atom is best classified as
A) an element
B) a compound
C) a homogeneous mixture
D) a heterogeneous mixture
A) an element
B) a compound
C) a homogeneous mixture
D) a heterogeneous mixture
A
4
Which statement best describes N2
A) it is an element
B) it is a compound
C) it is a homogeneous mixture
D) it is a heterogeneous mixture
E) both a & b
A) it is an element
B) it is a compound
C) it is a homogeneous mixture
D) it is a heterogeneous mixture
E) both a & b

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
5
A clear liquid is placed into a beaker and heated until it boils. After all of the liquid has evaporated, a white powder is found in the beaker. The original liquid is best classified as
A) an element
B) a compound
C) a homogeneous mixture
D) a heterogeneous mixture
A) an element
B) a compound
C) a homogeneous mixture
D) a heterogeneous mixture

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
6
A red solid is heated in an evacuated container until it completely vaporizes. When the vapor cools, it is found to be a sliver liquid. The mass of the silver liquid is less than that of the original solid. The red solid is best classified as
A) an element
B) a compound
C) a homogeneous mixture
D) a heterogeneous mixture
A) an element
B) a compound
C) a homogeneous mixture
D) a heterogeneous mixture

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
7
A sample of carotene is extracted from a carrot and found to contain 17.9 g of carbon and 2.1 g of hydrogen. A second carotene sample is synthesized in the lab and found to contain 10.0 g of carbon. How many grams of hydrogen are in the second sample?
A) 0.021 g
B) 0.940 g
C) 1.17 g
D) 2.00 g
E) 3.76 g
A) 0.021 g
B) 0.940 g
C) 1.17 g
D) 2.00 g
E) 3.76 g

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
8
Sodium chloride that is formed from a reaction of sodium vapor and chlorine gas is found to be 39.34% sodium by mass. A 4.86 g sample of sodium chloride that is purified from sea water contains how many grams of sodium?
A) 0.980 g
B) 1.91 g
C) 2.95 g
D) 4.46 g
E) there is no way to know because the compound is derived from a different source
A) 0.980 g
B) 1.91 g
C) 2.95 g
D) 4.46 g
E) there is no way to know because the compound is derived from a different source

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
9
Two elements X and Y combine to form two compounds with the following compositions  If compound 1 has the formula XY3 then a possible formula for compound 2 is
If compound 1 has the formula XY3 then a possible formula for compound 2 is
A) XY2
B) X2Y5
C) XY4
D) X2Yl9
E) XY5
 If compound 1 has the formula XY3 then a possible formula for compound 2 is
If compound 1 has the formula XY3 then a possible formula for compound 2 isA) XY2
B) X2Y5
C) XY4
D) X2Yl9
E) XY5

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
10
A compound is found to be 40.62% Si and 59.94% N. A possible formula for this compound is
A) Si2N
B) SiN2
C) SiN3
D) Si2N6
E) SiN3 and Si2N6 are both possible
A) Si2N
B) SiN2
C) SiN3
D) Si2N6
E) SiN3 and Si2N6 are both possible

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
11
You are trying to use percent mass of hydrogen to determine if a compound is epinephrine (C9H13NO3) or nor-epinephrine (C8H11NO3). What is the maximum error that is acceptable for your instrument in order to distinguish between these two compounds?
A) °0.5%
B) °1%
C) °2%
D) °5%
E) °10%
A) °0.5%
B) °1%
C) °2%
D) °5%
E) °10%

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
12
A 20.0 g a sample is composed of a mixture of Fe2O3 and Fe3O4. The mixture is found to contain 14.45 g of iron. What percent of the sample is Fe2O3?
A) 2%
B) 22%
C) 50%
D) 72%
E) 98%
A) 2%
B) 22%
C) 50%
D) 72%
E) 98%

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
13
In the presence of a catalyst carbon monoxide (CO) will reaction with oxygen (O2) to form carbon dioxide (CO2). How many liters of carbon dioxide can be formed from 1.5 L of carbon monoxide (with excess oxygen) if all the gases are held at the same temperature and pressure?
A) 0.15 L
B) 0.5 L
C) 0.75 L
D) 1.5 L
E) 3.0 L
A) 0.15 L
B) 0.5 L
C) 0.75 L
D) 1.5 L
E) 3.0 L

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
14
Gibbsite is an aluminum ore that is composed of 34.59% Al, 3.88% H, and 61.53% O. How much aluminum can be refined from 248.0 g of Gibbsite?
A) 7.09 g
B) 22.81 g
C) 45.59 g
D) 85.78 g
E) 152.6 g
A) 7.09 g
B) 22.81 g
C) 45.59 g
D) 85.78 g
E) 152.6 g

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
15
1.28 g of pure gold reacts with chlorine gas to produce 1.97 g of a red solid. A possible formula for the red solid is?
A) Au2Cl
B) AuCl
C) AuCl2
D) Au2Cl5
E) Au2Cl6
A) Au2Cl
B) AuCl
C) AuCl2
D) Au2Cl5
E) Au2Cl6

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
16
Methane gas (CH4) reacts with oxygen (O2) to produce water vapor and carbon dioxide. What volume of water (H2O) and carbon dioxide (CO2) will be formed from the complete reaction of 3 L of methane with oxygen if all the gases are held at the same temperature and pressure?
A) 3 L of H2O and 3 L of CO2
B) 6 L of H2O and 3 L of CO2
C) 3 L of H2O and 6 L of CO2
D) 6 L of H2O and 6 L of CO2
E) 3 L of H2O and 1.5 L of CO2
A) 3 L of H2O and 3 L of CO2
B) 6 L of H2O and 3 L of CO2
C) 3 L of H2O and 6 L of CO2
D) 6 L of H2O and 6 L of CO2
E) 3 L of H2O and 1.5 L of CO2

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
17
The element zinc has two naturally occurring isotopes. The natural abundances and isotopic masses are  The atomic mass of naturally occurring zinc is
The atomic mass of naturally occurring zinc is
A) 112.990
B) 113.582
C) 113.904
D) 114.582
E) 114.818
 The atomic mass of naturally occurring zinc is
The atomic mass of naturally occurring zinc isA) 112.990
B) 113.582
C) 113.904
D) 114.582
E) 114.818

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
18
The element antinomy has two naturally occurring isotopes. The natural abundances and isotopic masses are  The atomic mass of naturally occurring antinomy is
The atomic mass of naturally occurring antinomy is
A) 121.760
B) 121.904
C) 122.048
D) 122.904
E) 124.048
 The atomic mass of naturally occurring antinomy is
The atomic mass of naturally occurring antinomy isA) 121.760
B) 121.904
C) 122.048
D) 122.904
E) 124.048

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
19
How many neutrons are in a nucleus of a 235U atom?
A) 51
B) 92
C) 143
D) 194
E) 235
A) 51
B) 92
C) 143
D) 194
E) 235

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
20
Which has the greatest number of neutrons?
A) 238U
B) 237Np
C) 239Pu
D) 240Am
E) 240Cm
A) 238U
B) 237Np
C) 239Pu
D) 240Am
E) 240Cm

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
21
Which has the same number of neutrons as protons?
A) 1H
B) 19F
C) 32S
D) 40Ar
E) 80Se
A) 1H
B) 19F
C) 32S
D) 40Ar
E) 80Se

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
22
In Millikan's oil drop experiment, he was able to determine the charge of the electron because
A) each oil drop had only one electron
B) each oil drop contained an integer number of electrons
C) each oil drop had an identical charge
D) the oil drops were too small to be affected by gravity
E) the oil drops all fell at a constant speed
A) each oil drop had only one electron
B) each oil drop contained an integer number of electrons
C) each oil drop had an identical charge
D) the oil drops were too small to be affected by gravity
E) the oil drops all fell at a constant speed

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
23
In Rutherford's experiment scattering a particles off gold foil he
A) observed that a small fraction of the a particles were negatively charged.
B) observed that most of the a particles pass straight through the foil.
C) observed that a small fraction of the a particles scattered backwards from the foil.
D) both b & c
E) all of the above
A) observed that a small fraction of the a particles were negatively charged.
B) observed that most of the a particles pass straight through the foil.
C) observed that a small fraction of the a particles scattered backwards from the foil.
D) both b & c
E) all of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
24
Naturally occurring silicon is composed of three isotopes: 28Si (atomic mass 27.98), 29Si (atomic mass 28.98), and 30Si (atomic mass 29.97). The atomic mass of the naturally occurring isotope mixture is 28.0855. The relative percentages of 28Si, 29Si, and 30Si are
A) 99.7, 0.1, 0.2
B) 92.2, 4.7, 3.1
C) 90.2, 1.3, 8.5
D) 80.2, 10.8, 9.0
E) 50.4, 20.5, 29.1
A) 99.7, 0.1, 0.2
B) 92.2, 4.7, 3.1
C) 90.2, 1.3, 8.5
D) 80.2, 10.8, 9.0
E) 50.4, 20.5, 29.1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
25
Radon has seventeen isotopes ranging from 210Rd to 226Rd. What do all of these isotopes have in common?
A) the same number of electrons
B) the same number of protons
C) the same number of neutrons
D) a and b
E) b and c
A) the same number of electrons
B) the same number of protons
C) the same number of neutrons
D) a and b
E) b and c

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
26
What elemental isotope has 51 protons and 72 neutrons?
A) 21Ne
B) 51V
C) 72Ge
D) 123Sb
E) 123Te
A) 21Ne
B) 51V
C) 72Ge
D) 123Sb
E) 123Te

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
27
If the density of the nucleus of gold is approximately 1×1015 g cm-3 and solid gold has a density of 19.3 g cm-3 estimate the percentage of the volume of solid gold that is "occupied" by the nucleus?
A) 2×10-12 %
B) 2×10-8 %
C) 2×10-4 %
D) 2×10-2 %
E) 2%
A) 2×10-12 %
B) 2×10-8 %
C) 2×10-4 %
D) 2×10-2 %
E) 2%

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
28
10.3 g of an unknown compound is sealed in a tube with 25.8 g of hydrochloric acid. The tube is heated the compound completely dissolves. What is the mass of the contents of the tube after the reaction?
A) exactly 36.1 g
B) slightly more than 35.8 g
C) slightly less than 35.8 g
D) exactly 10 g
E) there is no way to know without more information about the reaction
A) exactly 36.1 g
B) slightly more than 35.8 g
C) slightly less than 35.8 g
D) exactly 10 g
E) there is no way to know without more information about the reaction

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
29
In a strange and alternative universe you repeat Millikan's oil drop experiment and measure the following charges on different oil drops  Using this give your best estimate for the unit of fundamental charge in this alternative universe
Using this give your best estimate for the unit of fundamental charge in this alternative universe
A) 1.2×10-17 C
B) 1.8×10-17 C
C) 3.6×10-17 C
D) 9.0×10-17 C
E) 1.2×10-16 C
 Using this give your best estimate for the unit of fundamental charge in this alternative universe
Using this give your best estimate for the unit of fundamental charge in this alternative universeA) 1.2×10-17 C
B) 1.8×10-17 C
C) 3.6×10-17 C
D) 9.0×10-17 C
E) 1.2×10-16 C

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
30
Chlorine has two naturally occurring isotopes 35Cl (atomic mass 34.97) and 37Cl (atomic mass of 36.96). If ions of naturally occurring Cl2 are detected in a mass spectrometer how many peaks should be observed?
A) one
B) two
C) three
D) four
E) five
A) one
B) two
C) three
D) four
E) five

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
31
Which nuclide has more protons than neutrons?
A)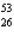 Fe
Fe
B)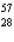 Ni
Ni
C)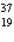 K
K
D)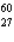 Co
Co
A)
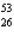 Fe
FeB)
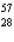 Ni
NiC)
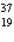 K
KD)
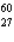 Co
Co
Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
32
Antimony has two naturally occurring isotopes with masses given in the table below. The relative atomic mass given for antimony on the periodic table is 121.75.  Which of the following is true?
Which of the following is true?
A) When a sample of antimony is placed in a mass spectrometer, one peak will be observed at the average relative atomic mass.
B) Two peaks will be observed and the 121Sb will be larger.
C) Two peaks will be observed and the 123Sb will be larger.
D) Three peaks will be observed at 120.9038, 121.75, and 122.90911.
E) Not enough information is given to answer the problem.
 Which of the following is true?
Which of the following is true?A) When a sample of antimony is placed in a mass spectrometer, one peak will be observed at the average relative atomic mass.
B) Two peaks will be observed and the 121Sb will be larger.
C) Two peaks will be observed and the 123Sb will be larger.
D) Three peaks will be observed at 120.9038, 121.75, and 122.90911.
E) Not enough information is given to answer the problem.

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
33
Distillation can be used to separate single phase solutions that are:
A) Compounds of different elements
B) Homogeneous mixtures of different compounds
C) Composed of elemental isotopes
D) Substances of different compounds
E) None of the above
A) Compounds of different elements
B) Homogeneous mixtures of different compounds
C) Composed of elemental isotopes
D) Substances of different compounds
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck


