Deck 6: Enzymes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/79
Play
Full screen (f)
Deck 6: Enzymes
1
The minimum amount of energy required to bring about a chemical reaction is called __________.
A) activation energy
B) enthalpy of reaction
C) free energy
D) standard free energy
E) transition state
A) activation energy
B) enthalpy of reaction
C) free energy
D) standard free energy
E) transition state
A
2
Which of the following is not a property of enzymes?
A) Capable of being regulated
B) Reaction rates high in comparison to uncatalyzed reaction
C) Highly specific
D) Side products of reactions are rare.
E) All of the above are correct.
A) Capable of being regulated
B) Reaction rates high in comparison to uncatalyzed reaction
C) Highly specific
D) Side products of reactions are rare.
E) All of the above are correct.
E
3
Consider the following reaction diagram. Which letter indicates the transition state? 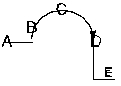
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E
C
4
In contrast to inorganic catalysts, enzymes have an intricately shaped surface called the __________.
A) substrate
B) cofactor
C) active site
D) apoenzyme
E) holoenzyme
A) substrate
B) cofactor
C) active site
D) apoenzyme
E) holoenzyme

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
5
The lock and key model of enzyme activity proposes that each __________.
A) enzyme can react with only a single substrate
B) enzyme has a cofactor that promotes the catalytic activity
C) substrate has a specific cofactor that binds it to the enzyme
D) enzyme binds a specific substrate because the active site and substrate have complementary structures.
E) Both A and B are correct.
A) enzyme can react with only a single substrate
B) enzyme has a cofactor that promotes the catalytic activity
C) substrate has a specific cofactor that binds it to the enzyme
D) enzyme binds a specific substrate because the active site and substrate have complementary structures.
E) Both A and B are correct.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
6
The synthesis of enzymes in response to changing metabolic needs is referred to as __________.
A) enzyme induction
B) allosteric regulation
C) negative feedback
D) zymogen activation
E) cooperative binding
A) enzyme induction
B) allosteric regulation
C) negative feedback
D) zymogen activation
E) cooperative binding

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is a coenzyme?
A) NADP+
B) Zn++
C) Cu++
D) Insulin
E) Oxytocin
A) NADP+
B) Zn++
C) Cu++
D) Insulin
E) Oxytocin

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
8
Alcohol dehydrogenase without NAD+ is called a __________.
A) apoenzyme
B) holoenzyme
C) substrate
D) cofactor
E) coenzyme
A) apoenzyme
B) holoenzyme
C) substrate
D) cofactor
E) coenzyme

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
9
The term synthetase is included in which class of enzymes?
A) Ligases
B) Hydrolases
C) Transferases
D) Lyases
E) Isomerases
A) Ligases
B) Hydrolases
C) Transferases
D) Lyases
E) Isomerases

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is not a type of oxidoreductase?
A) Peroxidase
B) Hydroxylase
C) Reductase
D) Dehydrogenase
E) Peptidase
A) Peroxidase
B) Hydroxylase
C) Reductase
D) Dehydrogenase
E) Peptidase

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following classes of enzymes catalyze reactions involving the cleavage of bonds by the addition of water?
A) Transferase
B) Hydrolase
C) Lyase
D) Ligase
E) Isomerase
A) Transferase
B) Hydrolase
C) Lyase
D) Ligase
E) Isomerase

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
12
Consider the following reaction data.
Alanylanine + water ? alanine
The reaction is __________ order overall.
A) zero
B) first
C) second
D) third
E) fourth
Alanylanine + water ? alanine
The reaction is __________ order overall.
A) zero
B) first
C) second
D) third
E) fourth

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
13
The steady state assumption states that if
K1 = the rate constant for ES formation
K2 = the rate constant for ES dissociation
K3 = the rate constant for product formation
A) k2 is highly negligible compared with k3
B) The rate of formation of ES is equal to the rate of its degradation over the course of the reaction.
C) The rate of formation of ES exceeds the rate of degradation over the course of the reaction.
D) k3 is negligible when compared to k2.
E) Product concentration at the beginning of the reaction is low.
K1 = the rate constant for ES formation
K2 = the rate constant for ES dissociation
K3 = the rate constant for product formation
A) k2 is highly negligible compared with k3
B) The rate of formation of ES is equal to the rate of its degradation over the course of the reaction.
C) The rate of formation of ES exceeds the rate of degradation over the course of the reaction.
D) k3 is negligible when compared to k2.
E) Product concentration at the beginning of the reaction is low.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
14
The expression of the Michaelis constant is equal to
A) (K2 + K3)/K1
B) (K2 + K1./K3
C) (K1 + K2)/K3
D) (K3 + K1) + K3
E) (K2/K1) + K3
Where
K1 = the rate constant for ES formation
K2 = the rate constant for ES dissociation
K3 = the rate constant for product formation
A) (K2 + K3)/K1
B) (K2 + K1./K3
C) (K1 + K2)/K3
D) (K3 + K1) + K3
E) (K2/K1) + K3
Where
K1 = the rate constant for ES formation
K2 = the rate constant for ES dissociation
K3 = the rate constant for product formation

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
15
Specific activity is defined as __________.
A) enzyme concentration that converts 1 mole of substrate to product per minute.
B) enzyme concentration that converts 1 mole of substrate to product per minute
C) the number of I.U. per mg of protein
D) the number of I. U. per gram of protein
E) enzyme concentration that converts 1 mm of substrate to product per minute.
A) enzyme concentration that converts 1 mole of substrate to product per minute.
B) enzyme concentration that converts 1 mole of substrate to product per minute
C) the number of I.U. per mg of protein
D) the number of I. U. per gram of protein
E) enzyme concentration that converts 1 mm of substrate to product per minute.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
16
In the Lineweaver-Burk double reciprocal plot the slope is equal to __________.
A) 1/[S]
B) 1/V
C) Km/Vmax
D) 1/Vmax
E) -1/Km
A) 1/[S]
B) 1/V
C) Km/Vmax
D) 1/Vmax
E) -1/Km

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
17
In the Lineweaver-Burk double reciprocal plot the vertical intercept is equal to __________.
A) 1/[S]
B) 1/V
C) Km/Vmax
D) 1/Vmax
E) -1/Km
A) 1/[S]
B) 1/V
C) Km/Vmax
D) 1/Vmax
E) -1/Km

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
18
In the Lineweaver-Burk double reciprocal plot the horizontal intercept is equal to __________.
A) 1/[S]
B) 1/V
C) Km/Vmax
D) 1/Vmax
E) -1/Km
A) 1/[S]
B) 1/V
C) Km/Vmax
D) 1/Vmax
E) -1/Km

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
19
In competitive inhibition, increasing the concentration of substrate __________
A) decreases the overall rate of the reaction
B) increases the overall rate of the reaction
C) is without effect
D) the observed effect depends on the inhibitor
E) competitive inhibitors do not affect the rate of the reaction
A) decreases the overall rate of the reaction
B) increases the overall rate of the reaction
C) is without effect
D) the observed effect depends on the inhibitor
E) competitive inhibitors do not affect the rate of the reaction

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following amino acids is capable of acting as a general acid or general base at physiological pH?
A) Glycine
B) Histidine
C) Tyrosine
D) Tryptophan
E) Proline
A) Glycine
B) Histidine
C) Tyrosine
D) Tryptophan
E) Proline

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following amino acids can participate in covalent catalysis?
A) Tryptophan
B) Alanine
C) Serine
D) Histidine
E) Both C and D are correct.
A) Tryptophan
B) Alanine
C) Serine
D) Histidine
E) Both C and D are correct.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is a feature of transition metals that makes them efficient cofactors?
A) Have a high concentration of positive charge
B) Can act as a Lewis acid
C) Have directed valences
D) Can exist as a variety of valence states
E) All of the above are correct.
A) Have a high concentration of positive charge
B) Can act as a Lewis acid
C) Have directed valences
D) Can exist as a variety of valence states
E) All of the above are correct.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
23
NADPH and NADH are coenzymes found in which class of enzymes?
A) Dehydrogenases
B) Ligases
C) Hydrolases
D) Transferases
E) Both C and D are correct.
A) Dehydrogenases
B) Ligases
C) Hydrolases
D) Transferases
E) Both C and D are correct.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is not present in the active site of alcohol dehydrogenase?
A) Zn++
B) Histidine
C) Cysteine
D) NAD+
E) Proline
A) Zn++
B) Histidine
C) Cysteine
D) NAD+
E) Proline

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
25
Enzyme control is accomplished in which of the following ways?
A) Genetic control
B) Covalent modification
C) Allosteric regulation
D) Compartmentation
E) All of the above are correct.
A) Genetic control
B) Covalent modification
C) Allosteric regulation
D) Compartmentation
E) All of the above are correct.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
26
Zymogens are a feature of what type of enzymatic control?
A) Genetic control
B) Covalent modification
C) Allosteric regulation
D) Compartmentation
E) Both B and C are correct.
A) Genetic control
B) Covalent modification
C) Allosteric regulation
D) Compartmentation
E) Both B and C are correct.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
27
Regulatory enzymes are a feature of what type of enzymatic control?
A) Genetic control
B) Covalent modification
C) Allosteric regulation
D) Compartmentation
E) Both B and C are correct.
A) Genetic control
B) Covalent modification
C) Allosteric regulation
D) Compartmentation
E) Both B and C are correct.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
28
Positive cooperativity is a feature of what type of enzymatic control?
A) Genetic control
B) Covalent modification
C) Allosteric regulation
D) Compartmentation
E) Both B and C are correct.
A) Genetic control
B) Covalent modification
C) Allosteric regulation
D) Compartmentation
E) Both B and C are correct.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
29
Segregation of biochemical pathways into different organelles in an example of which type of enzymatic regulation?
A) Genetic control
B) Covalent modification
C) Allosteric regulation
D) Compartmentation
E) Both B and C are correct.
A) Genetic control
B) Covalent modification
C) Allosteric regulation
D) Compartmentation
E) Both B and C are correct.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
30
Catalysts are effective because they __________.
A) decrease the rate of the reverse reaction
B) stabilize the transition state
C) decrease the activation energy of a reaction
D) increase the energy released during a reaction
E) Both B and C are correct.
A) decrease the rate of the reverse reaction
B) stabilize the transition state
C) decrease the activation energy of a reaction
D) increase the energy released during a reaction
E) Both B and C are correct.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
31
Enzymes act by __________.
A) decreasing the energy of activation of a reaction
B) increasing the energy of activation of a reaction
C) raising the temperature of a reaction
D) providing a surface to favorably orient the reactants
E) Both A and D are correct.
A) decreasing the energy of activation of a reaction
B) increasing the energy of activation of a reaction
C) raising the temperature of a reaction
D) providing a surface to favorably orient the reactants
E) Both A and D are correct.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
32
Enzyme studies are best carried out in __________.
A) dilute aqueous solution
B) highly concentrated solutions of the enzyme
C) highly concentrated solutions of the substrate
D) the presence of an inert crowding agent
E) the presence of a membrane
A) dilute aqueous solution
B) highly concentrated solutions of the enzyme
C) highly concentrated solutions of the substrate
D) the presence of an inert crowding agent
E) the presence of a membrane

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
33
Alcohol dehydrogenase is an example of which of the following classes of enzymes?
A) Oxidoreductases
B) Transferase
C) Hydrolase
D) Lyase
E) Isomerase
A) Oxidoreductases
B) Transferase
C) Hydrolase
D) Lyase
E) Isomerase

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is not an assumption of the law of mass action?
A) Forward reaction is linear.
B) Reverse reaction is linear.
C) System is homogenous.
D) Interacting molecules move randomly and independently of each other.
E) All are assumptions of the law of mass action.
A) Forward reaction is linear.
B) Reverse reaction is linear.
C) System is homogenous.
D) Interacting molecules move randomly and independently of each other.
E) All are assumptions of the law of mass action.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
35
Metabolons are defined as __________.
A) multifunction enzymes
B) complexes that channel product molecules from one active site to another
C) metabolic intermediates
D) effector molecules
E) rate of flow of metabolites from one point to another
A) multifunction enzymes
B) complexes that channel product molecules from one active site to another
C) metabolic intermediates
D) effector molecules
E) rate of flow of metabolites from one point to another

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
36
Metabolic flux is best defined as a __________.
A) lubrication enzyme that promotes the flow of reactants
B) rate of flow of metabolites from one point to another in a pathway
C) rate of a reaction
D) promoter molecule
E) Both A and D are correct.
A) lubrication enzyme that promotes the flow of reactants
B) rate of flow of metabolites from one point to another in a pathway
C) rate of a reaction
D) promoter molecule
E) Both A and D are correct.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
37
An enzyme without it cofactor is called __________.
A) coenzyme
B) apoenzyme
C) holoenzyme
D) noncatalytic
E) isoenzyme
A) coenzyme
B) apoenzyme
C) holoenzyme
D) noncatalytic
E) isoenzyme

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following amino acids cannot actively participate in a catalytic site?
A) Serine
B) Threonine
C) Tyrosine
D) Glycine
E) Glutamine
A) Serine
B) Threonine
C) Tyrosine
D) Glycine
E) Glutamine

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
39
In addition to serine and aspartate which of the following amino acids is a member of the serine triad?
A) Threonine
B) Tyrosine
C) Glutamine
D) Histidine
E) Glycine
A) Threonine
B) Tyrosine
C) Glutamine
D) Histidine
E) Glycine

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is not an important metal in biological systems?
A) Na+
B) K+
C) Mg++
D) Cu++
E) Ba++
A) Na+
B) K+
C) Mg++
D) Cu++
E) Ba++

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
41
The major site of alcohol detoxification is __________.
A) intestine
B) kidney
C) pancreas
D) liver
E) stomach
A) intestine
B) kidney
C) pancreas
D) liver
E) stomach

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
42
The main enzyme used to detoxify alcohol in humans is __________.
A) ADH1
B) ADH2
C) ADH3
D) ADH4
E) All of the above are correct.
A) ADH1
B) ADH2
C) ADH3
D) ADH4
E) All of the above are correct.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
43
Consider the following diagram. What constitutes the activation energy for the forward reaction? 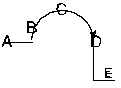
A) C-A
B) C-E
C) E-A
D) C-B
E) E-D

A) C-A
B) C-E
C) E-A
D) C-B
E) E-D

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following statements is not true of enzymes?
A) Increase the reaction rate
B) Obey the laws of thermodynamics
C) Catalyze the forward reaction only
D) Do not affect the position of equilibrium
E) Not consumed by the reaction
A) Increase the reaction rate
B) Obey the laws of thermodynamics
C) Catalyze the forward reaction only
D) Do not affect the position of equilibrium
E) Not consumed by the reaction

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
45
Hexokinase is an example of which class of enzymes?
A) Hydrolase
B) Lyase
C) Isomerase
D) Transferase
E) Ligase
A) Hydrolase
B) Lyase
C) Isomerase
D) Transferase
E) Ligase

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
46
Pyruvate carboxylase is an example of which class of enzymes?
A) Oxidoreductase
B) Transferase
C) Hydrolase
D) Ligase
E) Lyase
A) Oxidoreductase
B) Transferase
C) Hydrolase
D) Ligase
E) Lyase

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
47
What is meant by the term activation energy?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
48
Describe the term coenzyme. Give two examples.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
49
What is meant by the term turnover number?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
50
What is a noncompetitive inhibitor?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
51
What is a reaction intermediate?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
52
What is a proenzyme ?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
53
List four important properties of enzymes.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
54
Describe negative feedback inhibition.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
55
What properties of transition metals make them useful as enzyme cofactors?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
56
Explain why enzyme kinetics measurement are made at the start of a reaction.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
57
Describe the relationship between the law of mass action, solute concentration and effective concentration.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
58
Catalysts are believed to lower the activation energy of the transition state in a chemical reaction. How do they accomplish this task?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
59
Transition metals can act as Lewis acids. Explain.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
60
Is a reaction mechanism altered by the presence of a catalyst? Explain.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
61
Explain the difference between the energy of reaction and energy of activation.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
62
Mercuric ion and methyl alcohol are inhibitors of alcohol dehydrogenase. Explain.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
63
Describe the difference between reversible and irreversible inhibitors. Give an example of each.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
64
List three enzymatic dyads.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
65
How does an enzyme attain catalytic perfection?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
66
Water is often excluded from the active site of enzymes. Under these circumstances an amino nitrogen becomes much more nucleophilic. Explain.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
67
Can an activity coefficient ever be greater than one?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
68
Why do many enzymes require cofactors?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
69
Is catalytic perfection a function of concentration?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
70
Freeze drying is a useful technique for isolating enzymes. If the pH is not carefully controlled, however, the enzyme frequently becomes denatured. Explain.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
71
An enzyme is a large complex molecule much of which is seemingly uninvolved in the enzyme's catalytic function. Would it be possible to eliminate excess polypeptide segments and still retain activity?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
72
Some enzymes exist in the body as an inactive form (proenzymes) that are activated only when needed. What advantage do inactive precursors provide?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
73
Most enzymes are amino acid polymers. Would the replacement of the nitrogen in the peptide bond and side chains with oxygen be possible?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
74
How does aspirin inhibit the perception of pain?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
75
Catalase has a Km of 25 mM and a kcat of 4×107sec-1 with H2O2 as a substrate. Carbonic anhydrase has a Km of 26mM and a kcat of 4×105 sec-1. What does this data tell you about these two enzymes?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
76
The km and kcat for fumarase the enzyme that catalyzes the conversion of fumarate to malate are 5×10-6M and 8×102 s-1respectively. What does the data tell you about the operation of this enzyme in the citric acid cycle?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
77
Consider the following reaction along with its rate information
A + B C
 What is the overall rate expression for this reaction? What is the order of the reaction?
What is the overall rate expression for this reaction? What is the order of the reaction?
A + B C
 What is the overall rate expression for this reaction? What is the order of the reaction?
What is the overall rate expression for this reaction? What is the order of the reaction?
Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
78
Consider the following data for an enzyme-catalyzed hydrolysis reaction in the presence and absence of inhibitor I using a Michaelis-Menton plot determine Km for both inhibited and uninhibited reactions



Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
79
The table represents a specific substrate concentrations for an enzyme that displays classical Michaelis-Menten kinetics. Two sets of inhibitor data are also included. Determine the Km and Vmax for the uninhibited enzyme.



Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck


