Deck 3: Water: The Matrix of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/80
Play
Full screen (f)
Deck 3: Water: The Matrix of Life
1
How many hydrogen bonds can water form?
A) One
B) Two
C) Three
D) Four
E) Five
A) One
B) Two
C) Three
D) Four
E) Five
D
2
A hydrogen bond is best defined as:
A) A strong chemical bond between hydrogen and another element
B) A weak chemical bond between hydrogen and another element
C) A relatively strong electrostatic bond between hydrogen and oxygen or nitrogen
D) A weak electrostatic bond between hydrogen and oxygen or nitrogen
E) A bond between two hydrogens
A) A strong chemical bond between hydrogen and another element
B) A weak chemical bond between hydrogen and another element
C) A relatively strong electrostatic bond between hydrogen and oxygen or nitrogen
D) A weak electrostatic bond between hydrogen and oxygen or nitrogen
E) A bond between two hydrogens
C
3
Which of the following compounds are capable of hydrogen bonding with like molecules?
A) CH3CH2CH2CH2CH3
B) CH3CH2OCH2CH3
C) CH3NH2
D) HOCH2CH2OH
E) Both C and D are correct
A) CH3CH2CH2CH2CH3
B) CH3CH2OCH2CH3
C) CH3NH2
D) HOCH2CH2OH
E) Both C and D are correct
E
4
Which of the following is not an example of noncovalent interactions between molecules?
A) Salt bridges
B) Hydrogen bonds
C) Hydrophobic interactions
D) Ionic bonds
E) Chemical bonds
A) Salt bridges
B) Hydrogen bonds
C) Hydrophobic interactions
D) Ionic bonds
E) Chemical bonds

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following factors is responsible for the fact that water is a liquid at room temperature?
A) Covalent O-H bonds
B) Ionic bonds
C) Hydrogen bonds
D) Hydrophobic bonding between water molecules
E) The molecular weight of water
A) Covalent O-H bonds
B) Ionic bonds
C) Hydrogen bonds
D) Hydrophobic bonding between water molecules
E) The molecular weight of water

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following properties of water promotes the relatively constant climate of earth?
A) High heat of fusion
B) High surface tension
C) High heat of vaporization
D) High heat capacity
E) A, C, and D are all important
A) High heat of fusion
B) High surface tension
C) High heat of vaporization
D) High heat capacity
E) A, C, and D are all important

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following compounds is amphipathic?
A) H2O
B) CH3CH2CH2CH2CH3
C) HOOCCOOH
D) CH3CH2CH2CH2COOH
E) Both A and D are correct
A) H2O
B) CH3CH2CH2CH2CH3
C) HOOCCOOH
D) CH3CH2CH2CH2COOH
E) Both A and D are correct

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is the conjugate acid of the bicarbonate ion?
A)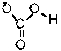
B)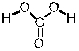
C)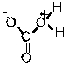
D)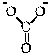
E)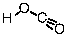
A)

B)

C)

D)
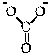
E)


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements is true of weak acids dissolved in water?
A) They are completely ionized in water
B) They are totally unionized in water
C) They are partially ionized in water
D) The dissociation constant is a function of pH
E) The dissociation constant is a function of solute concentration
A) They are completely ionized in water
B) They are totally unionized in water
C) They are partially ionized in water
D) The dissociation constant is a function of pH
E) The dissociation constant is a function of solute concentration

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
10
What is the pH of a solution where the concentration of hydrogen ions is 2 x 10-5 molar?
A) 5
B) 2.5
C) 2
D) 4.7
E) 5.3
A) 5
B) 2.5
C) 2
D) 4.7
E) 5.3

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following would not form a suitable buffer?
A) Acetic acid/ acetate
B) Carbonic acid/ bicarbonate
C) Bicarbonate/ carbonate
D) Hydrochloric acid/ chloride
E) Phosphoric acid/ dihydrogen phosphate
A) Acetic acid/ acetate
B) Carbonic acid/ bicarbonate
C) Bicarbonate/ carbonate
D) Hydrochloric acid/ chloride
E) Phosphoric acid/ dihydrogen phosphate

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
12
Buffering capacity is directly proportional to which of the following factors?
A) Molecular weight of the buffer
B) Concentration of the conjugate acid of the buffer
C) Concentration of the conjugate base of the buffer
D) Concentration of both components of the buffer
E) The acid or base to be buffered against
A) Molecular weight of the buffer
B) Concentration of the conjugate acid of the buffer
C) Concentration of the conjugate base of the buffer
D) Concentration of both components of the buffer
E) The acid or base to be buffered against

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
13
The sodium acetate/acetic acid buffer is 0.1 molar in sodium acetate and 0.5 molar in acetic acid. What is the concentration of the buffer?
A) 0.1 molar
B) 0.5 molar
C) 0.6 molar
D) 0.4 molar
E) Either A or B depending on whether acid or base is being added to the buffer.
A) 0.1 molar
B) 0.5 molar
C) 0.6 molar
D) 0.4 molar
E) Either A or B depending on whether acid or base is being added to the buffer.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
14
The pH of a solution that is 0.25 molar in acetic acid and 0.1 molar in sodium acetate is 4.36. What is the pKa of acetic acid?
A) 4.36
B) 4.76
C) 7.76
D) 3.76
E) 5.76
A) 4.36
B) 4.76
C) 7.76
D) 3.76
E) 5.76

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
15
The most important buffer in blood is __________.
A) Carbonate/bicarbonate buffer
B) Protein buffer
C) Phosphate buffer
D) Lactate buffer
E) Tartrate buffer
A) Carbonate/bicarbonate buffer
B) Protein buffer
C) Phosphate buffer
D) Lactate buffer
E) Tartrate buffer

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
16
When an individual breathes very rapidly, large amounts of carbon dioxide are exhaled. What effect does this have on blood pH?
A) Acidosis
B) Alkalosis
C) No change
D) First acidosis then rebounding alkalosis
E) First alkalosis then rebounding acidosos
A) Acidosis
B) Alkalosis
C) No change
D) First acidosis then rebounding alkalosis
E) First alkalosis then rebounding acidosos

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
17
Salt bridges in proteins are an example of _________.
A) Hydrogen bonds
B) Ionic interactions
C) Hydrophobic interactions
D) van der Waals forces
E) London dispersion forces
A) Hydrogen bonds
B) Ionic interactions
C) Hydrophobic interactions
D) van der Waals forces
E) London dispersion forces

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
18
What is the osmolarity of a solution that is 0.25 molar in trisodium phosphate?
A) 0.25 osmolar
B) 0.5 osmolar
C) 0.75 osmolar
D) 1.0 osmolar
E) None of the above is correct
A) 0.25 osmolar
B) 0.5 osmolar
C) 0.75 osmolar
D) 1.0 osmolar
E) None of the above is correct

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
19
Red blood cells are isotonic to a solution that is 0.9% sodium chloride. These same cells are _______________ to a solution that is 0.9% sodium sulfate.
A) Isotonic
B) Hypertonic
C) Hypotonic
D) Tonic
E) Nontonic
A) Isotonic
B) Hypertonic
C) Hypotonic
D) Tonic
E) Nontonic

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following molecules would have a dipole moment?
A) CCl4
B) CH3CH3
C) H2
D) CHCl3
E) I2
A) CCl4
B) CH3CH3
C) H2
D) CHCl3
E) I2

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following molecules would form a micelle?
A) NaCl
B) CH3COOH
C) CH3CH2CH2CH2CH2CH3
D) H3PO4
E) CH3(CH2)10COO- Na +
A) NaCl
B) CH3COOH
C) CH3CH2CH2CH2CH2CH3
D) H3PO4
E) CH3(CH2)10COO- Na +

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following molecules are weak acids?
A) HCl
B) CO3-2
C) HNO3
D) HCO3-
E) Both B and D
A) HCl
B) CO3-2
C) HNO3
D) HCO3-
E) Both B and D

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following ions would have the largest hydration sphere?
A) Li+
B) Na+
C) K+
D) Cs+
E) All would have the same size hydration sphere
A) Li+
B) Na+
C) K+
D) Cs+
E) All would have the same size hydration sphere

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
24
Hydration is best defined as
A) Covalent interaction between water and a solute
B) Noncovalent interaction between water and a solute
C) Salt formation in water
D) Ionization of a base to produce hydroxide ions
E) Both C and D are correct
A) Covalent interaction between water and a solute
B) Noncovalent interaction between water and a solute
C) Salt formation in water
D) Ionization of a base to produce hydroxide ions
E) Both C and D are correct

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
25
The noncovalent interaction between an amide and an alcohol would be which of the following?
A) Salt bridges
B) Hydrophobic
C) Hydrogen bonding
D) Both A and C are correct
E) All of the above are true
A) Salt bridges
B) Hydrophobic
C) Hydrogen bonding
D) Both A and C are correct
E) All of the above are true

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
26
Crenation would result when cells are placed in a ___________ solution.
A) Hypertonic
B) Hypotonic
C) Isotonic
D) Nontonic
E) Nonaqueous solvent
A) Hypertonic
B) Hypotonic
C) Isotonic
D) Nontonic
E) Nonaqueous solvent

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is not an important noncovalent interaction of living organisms?
A) Ionic interactions
B) Van der Waals interactions
C) Hydrogen bonds
D) Carbon hydrogen bonds
E) All are important
A) Ionic interactions
B) Van der Waals interactions
C) Hydrogen bonds
D) Carbon hydrogen bonds
E) All are important

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following molecules would have unusually high heat capacities?
A) Ammonia
B) Methane
C) Methyl alcohol
D) Benzene
E) Both A and C are correct
A) Ammonia
B) Methane
C) Methyl alcohol
D) Benzene
E) Both A and C are correct

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
29
The hybridization of the water molecule is:
A) Sp3
B) Sp2
C) Sp
D) Unhybridized
E) Planar
A) Sp3
B) Sp2
C) Sp
D) Unhybridized
E) Planar

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is the strongest type of non-covalent force?
A)Dipole-Dipole
B)Dipole-Induced Dipole
C)Induced Dipole-Induced Dipole
D)London dispersion forces
E)Hydrogen bond
A)Dipole-Dipole
B)Dipole-Induced Dipole
C)Induced Dipole-Induced Dipole
D)London dispersion forces
E)Hydrogen bond

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following ions would have the strongest tendency to form an ion pair with carboxylate groups on the surface of a protein?
A) Lithium
B) Sodium
C) Potassium
D) All have the same tendency
E) These ions do not associate with proteins
A) Lithium
B) Sodium
C) Potassium
D) All have the same tendency
E) These ions do not associate with proteins

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following species would form a buffer with HPO4-2?
A) H3PO4
B) H2PO4-1
C) CO3-2
D) All would form buffers with HPO4-2
E) Both A and B
A) H3PO4
B) H2PO4-1
C) CO3-2
D) All would form buffers with HPO4-2
E) Both A and B

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
33
Water can form how many hydrogen bonds?
A) One
B) Two
C) Three
D) Four
E) Five
A) One
B) Two
C) Three
D) Four
E) Five

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following solutions will be isotonic with 3M sucrose?
A) 3M NaCl
B) 1 M Sodium sulfate
C) 1.5 M Proline
D) 2 M Sodium chloride
E) 1 M Magnesium sulfate
A) 3M NaCl
B) 1 M Sodium sulfate
C) 1.5 M Proline
D) 2 M Sodium chloride
E) 1 M Magnesium sulfate

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following molecules is hydrophobic?
A) Sodium chloride
B) Ethyl alcohol
C) Hexane
D) Sucrose
E) Ammonia
A) Sodium chloride
B) Ethyl alcohol
C) Hexane
D) Sucrose
E) Ammonia

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
36
In biological systems buffers usually contain an excess of __________.
A) Weak base
B) Weak acid
C) The two components of the buffer are present in equal amounts
D) It would depend on the system
E) None of the above is correct
A) Weak base
B) Weak acid
C) The two components of the buffer are present in equal amounts
D) It would depend on the system
E) None of the above is correct

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
37
The tendency of nonpolar molecules to aggregate in a water medium is called ________.
A) Crenation
B) Hydrophobic effect
C) Hydrophillic effect
D) Micellular effect
E) Amphiatic
A) Crenation
B) Hydrophobic effect
C) Hydrophillic effect
D) Micellular effect
E) Amphiatic

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
38
The high heat of fusion of water is due to its _______________.
A) Hydrogen bonding
B) High surface tension
C) High heat of vaporization
D) High heat capacity
E) A, C, and D are all important
A) Hydrogen bonding
B) High surface tension
C) High heat of vaporization
D) High heat capacity
E) A, C, and D are all important

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is not a role of water in the body
A) Nutrient absorption
B) Nutrient transport
C) Waste product excretion
D) Temperature regulation
E) All are roles of water
A) Nutrient absorption
B) Nutrient transport
C) Waste product excretion
D) Temperature regulation
E) All are roles of water

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following species can form a buffer system?
A) Hydrochloric acid / Acetic acid
B) Acetic acid / Sodium chloride
C) Acetic acid / Ammonium chloride
D) Acetic acid/ Sodium acetate
E) Phosphoric acid / Sodium phosphate
A) Hydrochloric acid / Acetic acid
B) Acetic acid / Sodium chloride
C) Acetic acid / Ammonium chloride
D) Acetic acid/ Sodium acetate
E) Phosphoric acid / Sodium phosphate

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following are unusual properties of water that suit it to be the matrix
Of life?
A) Tetrahedral hybridization
B) Presence of oxygen
C) Thermal properties
D) Solvent characteristics
E) C and D
Of life?
A) Tetrahedral hybridization
B) Presence of oxygen
C) Thermal properties
D) Solvent characteristics
E) C and D

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
42
What percentage of the world's water is drinkable
A) 100%
B) 60%
C) 3%
D) 1%
E) 97%
A) 100%
B) 60%
C) 3%
D) 1%
E) 97%

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
43
The immediate effect of deforestation is
A) Drought
B) Erosion
C) Formation of grasslands
D) Formation of deserts
E) Increase in the water table
A) Drought
B) Erosion
C) Formation of grasslands
D) Formation of deserts
E) Increase in the water table

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is not an effect of hydrogen bonding on the
Physical properties of water?
A) Increased boiling point
B) Increased heat of fusion
C) Increased melting point
D) Increased heat of vaporization
E) All are effects of hydrogen bonding
Physical properties of water?
A) Increased boiling point
B) Increased heat of fusion
C) Increased melting point
D) Increased heat of vaporization
E) All are effects of hydrogen bonding

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
45
What is a salt bridge?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
46
Describe an isotonic solution.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
47
Define the term hydrophobic effect.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
48
Define the term alkalosis.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
49
How many hydrogen bonds can methanol form with water?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
50
Bicarbonate is the principle buffer of the blood and phosphate is the principle buffer within cells. Explain.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
51
What is the effect of hyperventilation on blood pH?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
52
Is it possible to prepare a buffer consisting of only carbonic acid and sodium carbonate?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
53
Many molecules are polar but they do not form significant hydrogen bonds. What is unusual about water that hydrogen bonding becomes possible?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
54
If the total concentration of a buffer is known (and not the individual concentrations of the weak acid and its conjugate base) can the pH of the buffer be calculated?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
55
What is the maximum number of hydrogen bonds that water can form?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
56
Describe how you would increase the buffering capacity of a 0.1 M acetate buffer.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
57
What is a micelle? How do micelles form?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
58
What is the hydronium ion?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
59
Pure sugars are often crystalline solids. Frequently, the process used to concentrate aqueous solutions of sugars produces syrups rather than crystals. Explain.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
60
Water forms stronger hydrogen bonds than ammonia. Suggest a reason for this.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
61
Water has been described as the universal solvent. If this was strictly true could life have arisen in this medium? Explain

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
62
Why can't seawater be used to water plants?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
63
The pH scale is valid only for water. Explain.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
64
Compounds such as the sugar trehalose are used as compatible solutes (i.e., water replacements) in desiccated organisms. What are the requirements for a substance that is to be used in this manner?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
65
When salt is spread on Jello (a mixture of gelatin and water), its consistency changes from a solid into a liquid. Explain.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
66
Explain why water has an unusually high heat capacity.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
67
Give an example of a molecule without an OH group that would be expected to have an unusually high heat capacity.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
68
Ammonia and water are both capable of forming hydrogen bonds. Why is water better at forming these bonds than ammonia?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
69
What is the major ionic structure for tyrosine at pH 7?
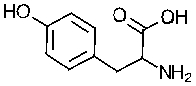 Tyrosine
Tyrosine
NH2 pKa = 9.11 COOH pKa 2.2 OH pKa = 10.07
 Tyrosine
TyrosineNH2 pKa = 9.11 COOH pKa 2.2 OH pKa = 10.07

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
70
In water fatty acids form a micelle with the COO- groups on the surface. The hydrophobic hydrocarbon tails are directed to the interior of the micelle. Explain.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
71
Explain why soaps that form micelles lower the surface tension of the water.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
72
If the osmotic pressure of a concentrated solution of sodium chloride is measured it is less than the calculated value. As the concentration of the sodium chloride is reduced the value of the osmotic pressure becomes closer and closer to the calculated value. Explain.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
73
Glucose is stored in cells as glycogen, an insoluble polymer. Hormone- triggered hydrolysis of glycogen produces glucose on demand. What advantages does this method of storage have for the cell?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
74
When plants are exposed to seawater they wilt and die. Explain.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
75
What is the osmolarity of a 1.3 M solution of sodium phosphate (Na3PO4)? Assume 85% ionization for this solution.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
76
What is the hydrogen ion concentration in a solution at pH 8.3?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
77
A solution that is 0.1M in acetic acid and 0.1 M in sodium acetate has a pH of 4.76. Determine the pKa of acetic acid. What is the Ka for acetic acid?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
78
Calculate the ratio of dihydrogen phosphate to hydrogen phosphate in blood at a pH 7.4. The Ka is 6.3 x10-8.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
79
What would be the pH of 1L of water if 1 ml of 1M HCl is added?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
80
Sketch the titration curve of the amino acid tyrosine starting with the following structure:
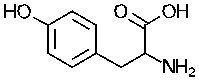 The pKa values are as follows: amino group 9.11, carboxyl group = 2.2 and side chain hydroxyl group = 10.07.
The pKa values are as follows: amino group 9.11, carboxyl group = 2.2 and side chain hydroxyl group = 10.07.
 The pKa values are as follows: amino group 9.11, carboxyl group = 2.2 and side chain hydroxyl group = 10.07.
The pKa values are as follows: amino group 9.11, carboxyl group = 2.2 and side chain hydroxyl group = 10.07.
Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck


