Deck 11: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/61
Play
Full screen (f)
Deck 11: Acids and Bases
1
Heartburn is caused by
A) a heart attack.
B) ingestion of acid.
C) acid in the stomach.
D) bBackflow of acid into the esophagus.
A) a heart attack.
B) ingestion of acid.
C) acid in the stomach.
D) bBackflow of acid into the esophagus.
D
2
Which of the following acids are an inorganic acid?
A) lactic acid
B) ascorbic acid
C) acetic acid
D) sulfuric acid
A) lactic acid
B) ascorbic acid
C) acetic acid
D) sulfuric acid
D
3
Which of the following is not a base?
A) sodium hydroxide
B) sodium chloride
C) sodium carbonate
D) sodium bicarbonate
A) sodium hydroxide
B) sodium chloride
C) sodium carbonate
D) sodium bicarbonate
B
4
Which of the following reactions describes the Brønsted-Lowry behavior of perchloric acid, HClO4?
A) HClO4(aq) + H2O(l) OH-(aq) + H2ClO4+aq)
B) HClO4(aq) + H2O(l) H3O+(aq) + ClO4-(aq)
C) HClO4(aq) + H2O(l) H3O+(aq) + ClO2-(aq) + O2(g)
D) 2 HClO4(aq) + H2O(l) H4O2+(aq) + 2ClO4-(aq)
A) HClO4(aq) + H2O(l) OH-(aq) + H2ClO4+aq)
B) HClO4(aq) + H2O(l) H3O+(aq) + ClO4-(aq)
C) HClO4(aq) + H2O(l) H3O+(aq) + ClO2-(aq) + O2(g)
D) 2 HClO4(aq) + H2O(l) H4O2+(aq) + 2ClO4-(aq)

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
5
In the Brønsted-Lowry model the acid
A) donates an OH.
B) accepts a H+.
C) donates a H+.
D) accepts an OH.
A) donates an OH.
B) accepts a H+.
C) donates a H+.
D) accepts an OH.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is a hydronium ion?
A) H+
B) OH-
C) H4O2+
D) H3O+
A) H+
B) OH-
C) H4O2+
D) H3O+

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following may be a Brønsted-Lowry base?
A) KOH
B) H2O
C) NH3
D) all of the above
A) KOH
B) H2O
C) NH3
D) all of the above

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following common drugs acts as a Brønsted-Lowry base?
A)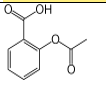
B)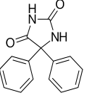
C)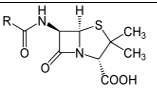
D)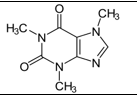
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following reactions shows the Brønsted-Lowry base dissociation of sodium hydroxide?
A) NaOH(aq) + H2O(l) Na+(aq) + OH-(aq) + H2O(l)
B) KOH(s) K+(aq) + OH-(aq)
C) NaOH(aq) + H+(aq) NaOH2+(aq)
D) NaOH(aq) + OH-(aq) Na(OH)2-(aq)
A) NaOH(aq) + H2O(l) Na+(aq) + OH-(aq) + H2O(l)
B) KOH(s) K+(aq) + OH-(aq)
C) NaOH(aq) + H+(aq) NaOH2+(aq)
D) NaOH(aq) + OH-(aq) Na(OH)2-(aq)

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
10
What is the net ionic equation for the reaction of nitric acid, HNO3, and potassium hydroxide, KOH?
A) HNO3(aq) + KOH(aq) H2O(l) + KNO3(aq)
B) H+(aq) + NO3-(aq) + K+(aq) + OH-(aq) H2O(l) + NO3-(aq) + K+(aq)
C) H+(aq) + OH-(aq) H2O(l)
D) HNO3(aq) + KOH(aq) H2O(l) + K+(aq) + NO3-(aq)
A) HNO3(aq) + KOH(aq) H2O(l) + KNO3(aq)
B) H+(aq) + NO3-(aq) + K+(aq) + OH-(aq) H2O(l) + NO3-(aq) + K+(aq)
C) H+(aq) + OH-(aq) H2O(l)
D) HNO3(aq) + KOH(aq) H2O(l) + K+(aq) + NO3-(aq)

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
11
Balance the equation for the neutralization of aluminum hydroxide, Al(OH)3, with HCl.
A) Al(OH)3(aq) + HCl(aq) H2O(l) + AlCl3(aq)
B) Al(OH)3(aq) + 2HCl(aq) 2H2O(l) + AlCl3(aq)
C) Al(OH)3(aq) + 3HCl(aq) 3H2O(l) + AlCl3(aq)
D) Al(OH)3(aq) + 4HCl(aq) 4H2O(l) + AlCl3(aq)
A) Al(OH)3(aq) + HCl(aq) H2O(l) + AlCl3(aq)
B) Al(OH)3(aq) + 2HCl(aq) 2H2O(l) + AlCl3(aq)
C) Al(OH)3(aq) + 3HCl(aq) 3H2O(l) + AlCl3(aq)
D) Al(OH)3(aq) + 4HCl(aq) 4H2O(l) + AlCl3(aq)

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
12
Balance the equation for the neutralization of calcium hydroxide, Ca(OH)2, and sulfuric acid, H2SO4.
A) Ca(OH)2(aq) + H2SO4(aq) 2H2O(l) + CaSO4(s)
B) Ca(OH)2(aq) + 2H2SO4(aq) 2H2O(l) + CaSO4(s)
C) 2Ca(OH)2(aq) + H2SO4(aq) 2H2O(l) + CaSO4(s)
D) 3Ca(OH)2(aq) + 2H2SO4(aq) H2O(l) + CaSO4(s)
A) Ca(OH)2(aq) + H2SO4(aq) 2H2O(l) + CaSO4(s)
B) Ca(OH)2(aq) + 2H2SO4(aq) 2H2O(l) + CaSO4(s)
C) 2Ca(OH)2(aq) + H2SO4(aq) 2H2O(l) + CaSO4(s)
D) 3Ca(OH)2(aq) + 2H2SO4(aq) H2O(l) + CaSO4(s)

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
13
Nitric acid is a strong acid and dissociates in water. What species are present in the aqueous solution of 0.1 M HNO3?
A) HNO3 only
B) primarily HNO3 but some H3O+ and NO3-
C) primarily H3O+ and NO3- but some HNO3
D) H3O+ and NO3- only
A) HNO3 only
B) primarily HNO3 but some H3O+ and NO3-
C) primarily H3O+ and NO3- but some HNO3
D) H3O+ and NO3- only

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
14
Lactic acid, CH3CH(OH)CO2H, is a weak acid that dissociates in water according to the equation CH3CH(OH)CO2H(aq) + H2O(l) CH3CH(OH)CO2-(aq) + H3O+(aq). What species are in the aqueous solution of 0.1 M lactic acid?
A) CH3CH(OH)CO2H only
B) CH3CH(OH)CO2H primarily but some H3O+ and CH3CH(OH)CO2-
C) H3O+ and CH3CH(OH)CO2- only
D) H3O+ and CH3CH(OH)CO2- primarily but some CH3CH(OH)CO2H
A) CH3CH(OH)CO2H only
B) CH3CH(OH)CO2H primarily but some H3O+ and CH3CH(OH)CO2-
C) H3O+ and CH3CH(OH)CO2- only
D) H3O+ and CH3CH(OH)CO2- primarily but some CH3CH(OH)CO2H

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
15
A solution of an acid exhibits very good electrical conductivity. It is a
A) weak acid.
B) strong acid.
C) covalent molecule.
D) all of the above
A) weak acid.
B) strong acid.
C) covalent molecule.
D) all of the above

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the solutions below will be highly conductive of electricity?
A) 0.1 M acetic acid, CH3CO2H
B) 0.1 M formic acid, CHO2H
C) 0.1 M hydrochloric acid, HCl
D) 0.1 M lactic acid, CH3CH(OH)CO2H
A) 0.1 M acetic acid, CH3CO2H
B) 0.1 M formic acid, CHO2H
C) 0.1 M hydrochloric acid, HCl
D) 0.1 M lactic acid, CH3CH(OH)CO2H

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is a true statement about an aqueous solution of a base when describing it as strong?
A) It is a good conductor of electricity.
B) Much of the unionized base exists in the solution.
C) It is a poor conductor of electricity.
D) none of the above
A) It is a good conductor of electricity.
B) Much of the unionized base exists in the solution.
C) It is a poor conductor of electricity.
D) none of the above

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
18
The acidity of formic acid, CHO2H, results from
A) CHO2H.
B) CHO2.
C) H2O.
D) H3O+.
A) CHO2H.
B) CHO2.
C) H2O.
D) H3O+.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
19
The conjugate base of HCl is
A) H2Cl+.
B) OH.
C) H3O+.
D) Cl.
A) H2Cl+.
B) OH.
C) H3O+.
D) Cl.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
20
What is the conjugate base of ammonium, NH4+?
A) OH
B) H2O
C) NH3
D) NH52+
A) OH
B) H2O
C) NH3
D) NH52+

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
21
What are the relative proportions of reactants to products for the dissociation of the weak base trimethylamine? (CH3)3N(aq) + H2O(l) (CH3)3NH+(aq) + OH-(aq)
A) The reaction consists of 100% of the reactant (CH3)3N.
B) The reaction consists of some of the reactant (CH3)3N but mostly the products (CH3)3NH+ and OH.
C) The reaction consists of mostly the reactant (CH3)3N but some of the products (CH3)3NH+ and OH.
D) The reaction consists of 100% of the products (CH3)3NH+ and OH.
A) The reaction consists of 100% of the reactant (CH3)3N.
B) The reaction consists of some of the reactant (CH3)3N but mostly the products (CH3)3NH+ and OH.
C) The reaction consists of mostly the reactant (CH3)3N but some of the products (CH3)3NH+ and OH.
D) The reaction consists of 100% of the products (CH3)3NH+ and OH.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
22
The bisulfite ion (HSO3-) is a weak acid. What is the missing species in the reversible dissociation of bisulfite in aqueous solution? HSO3-(aq) + H2O(l) H3O+(aq) + ?
A) SO32-
B) H2SO3
C) H2S + O2
D) SO2 + O2
A) SO32-
B) H2SO3
C) H2S + O2
D) SO2 + O2

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
23
What is the concentration of H3O+ ion in pure water?
A) 1 10-14 M
B) 2 10-14 M
C) 1 107 M
D) 1 10-7 M
A) 1 10-14 M
B) 2 10-14 M
C) 1 107 M
D) 1 10-7 M

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
24
What is the concentration of OH- ion in pure water?
A) 1 10-14 M
B) 2 10-14 M
C) 1 107 M
D) 1 10-7 M
A) 1 10-14 M
B) 2 10-14 M
C) 1 107 M
D) 1 10-7 M

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
25
At 25°C the term [H3O+][OH-] equals
A) 1 10-7.
B) 2 10-14.
C) 1 1014.
D) 1 10-14.
A) 1 10-7.
B) 2 10-14.
C) 1 1014.
D) 1 10-14.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
26
In pure water which is in greater concentration H3O+ or OH-?
A) [H3O+] > [OH-]
B) [H3O+] < [OH-]
C) [H3O+] = [OH-]
D) It varies depending upon temperature.
A) [H3O+] > [OH-]
B) [H3O+] < [OH-]
C) [H3O+] = [OH-]
D) It varies depending upon temperature.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
27
In a dilute solution of base at 25°C, the concentration of H3O+ is 1 10-9 M. Calculate the concentration of OH- ions.
A) 1 10-9 M
B) 1 10-7 M
C) 1 10-5 M
D) 1 10-2 M
A) 1 10-9 M
B) 1 10-7 M
C) 1 10-5 M
D) 1 10-2 M

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
28
In a dilute solution of acid at 25°C, the concentration of H3O+ is 1 10-3 M. What is the concentration of OH- ions?
A) 1 1011 M
B) 1 10-4 M
C) 1 10-7 M
D) 1 10-11 M
A) 1 1011 M
B) 1 10-4 M
C) 1 10-7 M
D) 1 10-11 M

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following solutions is acidic?
A) [OH-] = 1 10-8 M
B) [OH-] = 1 10-7 M
C) [OH-] = 1 10-5 M
D) [OH-] = 1 10-2 M
A) [OH-] = 1 10-8 M
B) [OH-] = 1 10-7 M
C) [OH-] = 1 10-5 M
D) [OH-] = 1 10-2 M

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following solutions is basic?
A) [H3O+] = 1 10-8 M
B) [H3O+] = 1 10-7 M
C) [H3O+] = 1 10-5 M
D) [H3O+] = 1 10-3 M
A) [H3O+] = 1 10-8 M
B) [H3O+] = 1 10-7 M
C) [H3O+] = 1 10-5 M
D) [H3O+] = 1 10-3 M

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
31
At 25°C the concentration of H3O+ in a solution is 1 10-2 M. What is the pH?
A) -2
B) 0.5
C) 0.01
D) 2
A) -2
B) 0.5
C) 0.01
D) 2

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
32
At 25°C the concentration of OH- in a solution is 1 10-8 M. What is the pH of the solution?
A) 8
B) 6
C) -8
D) -6
A) 8
B) 6
C) -8
D) -6

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
33
Milk has a H3O+ concentration of 1 10-6 M. What is the pH of the milk?
A) 5
B) 6
C) 7
D) 8
A) 5
B) 6
C) 7
D) 8

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
34
Perchloric acid (HClO4) is a strong acid. What is the pH of a 0.0001 M solution of this acid?
A) 4
B) 3
C) 5
D) 7
A) 4
B) 3
C) 5
D) 7

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
35
Black coffee has a pH of 5.0. What is the concentration of H3O+ in the coffee?
A) 1 10-5 M
B) 1 10-9 M
C) 1 10-7 M
D) 1 10-11 M
A) 1 10-5 M
B) 1 10-9 M
C) 1 10-7 M
D) 1 10-11 M

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
36
Lemon juice has a pH of 2.0. What is the concentration of H3O+ in the juice?
A) 1 10-2 M
B) 1 10-7 M
C) 1 10-5 M
D) 1 10-12 M
A) 1 10-2 M
B) 1 10-7 M
C) 1 10-5 M
D) 1 10-12 M

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
37
What is most important in the release of gastric acid in the stomach?
A) histamine
B) H2 receptor
C) proton pump
D) H2 receptor blockers
A) histamine
B) H2 receptor
C) proton pump
D) H2 receptor blockers

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following drugs is responsible for reducing the production of acid in the stomach?
A) antacid
B) histamine
C) H2 receptor blockers
D) proton pump
A) antacid
B) histamine
C) H2 receptor blockers
D) proton pump

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
39
Acid reflux disease (GERD) is caused by
A) generation of acid in the esophagus.
B) flow of acid from the esophagus to the stomach.
C) flow of acid from the stomach to the esophagus.
D) acid inflaming the stomach lining.
A) generation of acid in the esophagus.
B) flow of acid from the esophagus to the stomach.
C) flow of acid from the stomach to the esophagus.
D) acid inflaming the stomach lining.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
40
Acid is produced in the stomach by
A) histamine.
B) H2 receptors.
C) proton pump.
D) H. pylori.
A) histamine.
B) H2 receptors.
C) proton pump.
D) H. pylori.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following mixtures of chemical substances would be a buffer?
A) HCO3- and H2O
B) H2CO3 and H2O
C) H2CO3 and HCO3-
D) HCO3- and H3O+
A) HCO3- and H2O
B) H2CO3 and H2O
C) H2CO3 and HCO3-
D) HCO3- and H3O+

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
42
A carbonic acid (H2CO3) and bicarbonate (HCO3-) forms a buffer in the blood stream. Excess acid in the blood stream causes the
A) pH to rise dramatically.
B) carbonic acid to be consumed with very little change in pH.
C) bicarbonate to be consumed with very little change in pH.
D) pH to drop dramatically.
A) pH to rise dramatically.
B) carbonic acid to be consumed with very little change in pH.
C) bicarbonate to be consumed with very little change in pH.
D) pH to drop dramatically.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
43
A buffer could be prepared by mixing lactic acid with
A) HCl.
B) sodium acetate.
C) sodium lactate.
D) ammonium chloride.
A) HCl.
B) sodium acetate.
C) sodium lactate.
D) ammonium chloride.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
44
To produce a buffer from a weak acid, you would add
A) water.
B) its conjugate base.
C) another weak acid.
D) a salt.
A) water.
B) its conjugate base.
C) another weak acid.
D) a salt.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
45
The mixture of a weak acid and its conjugate base produces
A) a salt.
B) a strong acid and strong base.
C) water.
D) a buffer.
A) a salt.
B) a strong acid and strong base.
C) water.
D) a buffer.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
46
Individuals who experience frequent acid reflux have a condition called ____________________.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
47
Because acetic acid can dissociate into hydronium ion and acetate ion and then the acetate ion can accept a proton from a hydronium ion, the reaction is ____________________.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
48
Acetate is the ____________________ of acetic acid.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
49
When the relative amounts of reactant and product are in balance, the reaction has reached ____________________.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
50
The conjugate base of HSO4- is ____________________.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
51
A mixture of chemical substances that resists change in pH is a ____________________.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
52
The conjugate base of carbonic acid, H2CO3, is ____________________.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
53
Show the reaction that demonstrates trimethylamine, (CH3)3NH2 as a Brønsted-Lowry base.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
54
Iron(III) ion, Fe3+ produces acidic aqueous solutions. Why can it not be a Brønsted-Lowry acid?

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
55
Why is a weak acid, such as acetic acid, a weak conductor of electricity?

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
56
Chloride, Cl-, is the conjugate base of the strong acid HCl. Acetate, CH3CO2-, is the conjugate base of the weak acid acetic acid, CH3CO2H. What is the relative strength of these two bases? Explain your answer.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
57
What is the conjugate acid of OH-? Describe and explain the relative strength of each.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
58
Describe the action of a carbonic acid (H2CO3) and bicarbonate (HCO3-) buffer when a base is introduced.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
59
Describe the action of a carbonic acid (H2CO3) and bicarbonate (HCO3-) buffer when an acid is introduced.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
60
How would you prepare a buffer?

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
61
Describe the purpose of buffers in the blood stream.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck


