Deck 9: Molecules and Ions in Solution
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/58
Play
Full screen (f)
Deck 9: Molecules and Ions in Solution
1
Select the true statement.
A) All vitamins are water-soluble.
B) All vitamins are fat-soluble.
C) Vitamins do not dissolve in either water or fat.
D) Some vitamins dissolve in water; others dissolve in fat.
A) All vitamins are water-soluble.
B) All vitamins are fat-soluble.
C) Vitamins do not dissolve in either water or fat.
D) Some vitamins dissolve in water; others dissolve in fat.
D
2
Select the correct statement about an aqueous solution.
A) It is a heterogeneous mixture.
B) Water is the solute.
C) Water is the solvent.
D) The solvent can be any substance.
A) It is a heterogeneous mixture.
B) Water is the solute.
C) Water is the solvent.
D) The solvent can be any substance.
C
3
Forcing methane into water using pressure causes which of the following?
A) hydrogen bonding between methane and water
B) attraction of methane to water by dispersion forces
C) an increase in entropy since the water forms cage-like clathrates
D) the formation of water ice
A) hydrogen bonding between methane and water
B) attraction of methane to water by dispersion forces
C) an increase in entropy since the water forms cage-like clathrates
D) the formation of water ice
C
4
Select the true statement about hydrophobic molecules.
A) They are nonpolar.
B) They always contain carbon atoms
C) They are polar.
D) They may form hydrogen bonds with other molecules.
A) They are nonpolar.
B) They always contain carbon atoms
C) They are polar.
D) They may form hydrogen bonds with other molecules.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following would be expected to be soluble in water?
A) CH₃CH₃
B) CH₂CH₂
C) CH₃CH₂OH
D) CH₃CH₂OCH₂CH₂CH₃
A) CH₃CH₃
B) CH₂CH₂
C) CH₃CH₂OH
D) CH₃CH₂OCH₂CH₂CH₃

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
6
Why is methanol, CH₃OH, soluble in water?
A) It interacts with water using dispersion forces.
B) It has an -OH group that can hydrogen bond with water.
C) It is small and can fit between water molecules.
D) It forms ions when dissolved in water that interact strongly with water.
A) It interacts with water using dispersion forces.
B) It has an -OH group that can hydrogen bond with water.
C) It is small and can fit between water molecules.
D) It forms ions when dissolved in water that interact strongly with water.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
7
Complete the following sentence: The dispersion forces
A) become weaker as the molecule gets larger.
B) become stronger as the molecule gets larger.
C) are equal for all molecules regardless of size.
D) cannot be predicted from the size of the molecule.
A) become weaker as the molecule gets larger.
B) become stronger as the molecule gets larger.
C) are equal for all molecules regardless of size.
D) cannot be predicted from the size of the molecule.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
8
What is the dominant attractive force between nonpolar molecules?
A) dispersion forces
B) hydrogen bonding
C) polar-polar interaction
D) gravity
A) dispersion forces
B) hydrogen bonding
C) polar-polar interaction
D) gravity

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
9
Select the correct statement about the C-H hydrogen bonds in methane, CH₄.
A) The C-H bonds in methane may participate in hydrogen bonding with water.
B) The C-H bonds in methane are polar.
C) The C-H bonds in methane are nonpolar.
D) There is a very large difference in electronegativity between the carbon atom and hydrogen atom.
A) The C-H bonds in methane may participate in hydrogen bonding with water.
B) The C-H bonds in methane are polar.
C) The C-H bonds in methane are nonpolar.
D) There is a very large difference in electronegativity between the carbon atom and hydrogen atom.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
10
When methane is forced into liquid water, a clathrate forms. The clathrate is a
A) hydrogen bond between water and methane.
B) cage of water molecules that surrounds the methane molecules.
C) cage of methane molecules that surrounds the water molecules.
D) pocket of methane gas within the water.
A) hydrogen bond between water and methane.
B) cage of water molecules that surrounds the methane molecules.
C) cage of methane molecules that surrounds the water molecules.
D) pocket of methane gas within the water.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following examples describes an increase in entropy?
A) stacking wooden blocks
B) making sand castles
C) organizing a closet
D) dropping a stack of organized papers
A) stacking wooden blocks
B) making sand castles
C) organizing a closet
D) dropping a stack of organized papers

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
12
When methane is added to water, the formation of a clathrate
A) decreases the entropy of the system.
B) increases the entropy of the system.
C) releases energy from the system.
D) does not change the entropy of the system.
A) decreases the entropy of the system.
B) increases the entropy of the system.
C) releases energy from the system.
D) does not change the entropy of the system.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
13
Forcing methane into water causes
A) a release of energy from the system.
B) no change in energy for the system.
C) an expenditure of energy on the system.
D) energy to be converted to matter.
A) a release of energy from the system.
B) no change in energy for the system.
C) an expenditure of energy on the system.
D) energy to be converted to matter.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
14
When two or more nonpolar molecules are added to water, the hydrophobic effect causes the nonpolar molecule to
A) disperse throughout the water.
B) bind strongly with the water.
C) aggregate together.
D) evaporate from the water.
A) disperse throughout the water.
B) bind strongly with the water.
C) aggregate together.
D) evaporate from the water.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
15
The hydrophobic interaction between nonpolar molecules in water does which of the following?
A) shields some of the nonpolar molecules' surface from the surrounding water
B) increases the entropy penalty for forcing the water into a more orderly state
C) exposes more of the nonpolar molecules' surface to the surrounding water
D) disperses the nonpolar molecules throughout the solvent water
A) shields some of the nonpolar molecules' surface from the surrounding water
B) increases the entropy penalty for forcing the water into a more orderly state
C) exposes more of the nonpolar molecules' surface to the surrounding water
D) disperses the nonpolar molecules throughout the solvent water

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
16
A substance that is water soluble is considered
A) lypophilic.
B) hydrophilic.
C) hydrophobic.
D) polar.
A) lypophilic.
B) hydrophilic.
C) hydrophobic.
D) polar.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
17
What makes methanol, CH₃OH, easily dissolve in water?
A) C-H bond polarity
B) The carbon atom can interact with the water's hydrogen-bonding network.
C) The C-H group can incorporate into the water's hydrogen-bonding network.
D) The O-H group can incorporate into the water's hydrogen-bonding network.
A) C-H bond polarity
B) The carbon atom can interact with the water's hydrogen-bonding network.
C) The C-H group can incorporate into the water's hydrogen-bonding network.
D) The O-H group can incorporate into the water's hydrogen-bonding network.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
18
Which pair of molecules will most likely interact to form an attraction between them?
A) CH₄ and CH₃OH
B) H2O and CH₃OH
C) CH₄ and H₂O
D) none of the above (None of the pairs can interact to form an attraction.)
A) CH₄ and CH₃OH
B) H2O and CH₃OH
C) CH₄ and H₂O
D) none of the above (None of the pairs can interact to form an attraction.)

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following hydrocarbons would have the highest boiling point?
A) CH₄
B) CH₃CH₃
C) CH₃CH₂CH₃
D) CH₃CH2CH₂CH₃
A) CH₄
B) CH₃CH₃
C) CH₃CH₂CH₃
D) CH₃CH2CH₂CH₃

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
20
What type of attractive force allows hydrocarbons to exist as a liquid?
A) dipole-dipole
B) hydrogen bonding
C) dispersion forces
D) gravity
A) dipole-dipole
B) hydrogen bonding
C) dispersion forces
D) gravity

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
21
Dispersion forces in hydrocarbons increase with
A) increasing size of the hydrocarbon.
B) decreasing size of the hydrocarbon.
C) magnitude of permanent dipole of the hydrocarbon.
D) number of O-H groups.
A) increasing size of the hydrocarbon.
B) decreasing size of the hydrocarbon.
C) magnitude of permanent dipole of the hydrocarbon.
D) number of O-H groups.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
22
An instantaneous dipole in a hydrocarbon __________ dipole in a neighboring hydrocarbon.
A) induces a temporary
B) induces a permanent
C) inhibits a temporary
D) does not affect a
A) induces a temporary
B) induces a permanent
C) inhibits a temporary
D) does not affect a

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following molecules would be expected to be most soluble in the hydrocarbon hexane?
A) CH₃OH
B) CH₃CH₂OH
C) CH₃CH₂CH₂OH
D) CH₃CH₂CH₂CH₂OH
A) CH₃OH
B) CH₃CH₂OH
C) CH₃CH₂CH₂OH
D) CH₃CH₂CH₂CH₂OH

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
24
What forms adipose tissue?
A) muscle protein
B) a collection of cells that store hexane
C) a collection of cells that store glycerol
D) a collection of cells that store triglycerides
A) muscle protein
B) a collection of cells that store hexane
C) a collection of cells that store glycerol
D) a collection of cells that store triglycerides

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following vitamins would be fat-soluble?
A)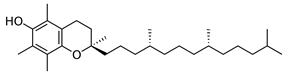
B)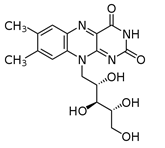
C)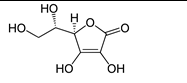
D)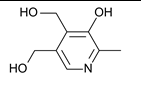
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following vitamins would be water-soluble?
A)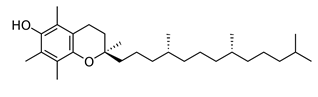
B)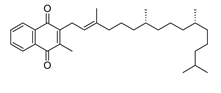
C)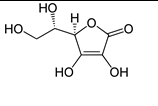
D)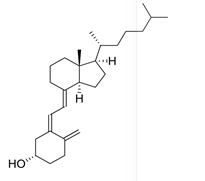
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
27
Select the false statement about water-soluble vitamins.
A) Even only one O-H group in a vitamin will ensure it is water soluble.
B) Water-soluble vitamins must contain sufficient polar groups, such as O-H, to offset hydrophobic properties.
C) Two water-soluble vitamins may differ in their solubility in water.
D) Even water-soluble vitamins will contain some hydrophobic parts.
A) Even only one O-H group in a vitamin will ensure it is water soluble.
B) Water-soluble vitamins must contain sufficient polar groups, such as O-H, to offset hydrophobic properties.
C) Two water-soluble vitamins may differ in their solubility in water.
D) Even water-soluble vitamins will contain some hydrophobic parts.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the statements best describes the USDA Recommended Dietary Allowance (RDA) for vitamins?
A) The RDA for fat-soluble vitamins is typically higher than that for water-soluble vitamins.
B) The RDA for fat-soluble vitamins is typically lower than that for water-soluble vitamins.
C) The RDA for fat-soluble vitamins is typically similar to that for water-soluble vitamins.
D) There is no RDA for vitamins.
A) The RDA for fat-soluble vitamins is typically higher than that for water-soluble vitamins.
B) The RDA for fat-soluble vitamins is typically lower than that for water-soluble vitamins.
C) The RDA for fat-soluble vitamins is typically similar to that for water-soluble vitamins.
D) There is no RDA for vitamins.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
29
Why may fat-soluble vitamins become toxic?
A) Because they are composed primarily of hydrophobic parts, they are naturally more toxic.
B) They accumulate in fat tissues and are not readily excreted from the body.
C) The transport molecules required to move them throughout the body are toxic.
D) They form solids in the blood stream and block blood flow.
A) Because they are composed primarily of hydrophobic parts, they are naturally more toxic.
B) They accumulate in fat tissues and are not readily excreted from the body.
C) The transport molecules required to move them throughout the body are toxic.
D) They form solids in the blood stream and block blood flow.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
30
The formation of a micelle when detergent is placed into water is considered
A) crystallization.
B) condensation.
C) precipitation.
D) self-assembly.
A) crystallization.
B) condensation.
C) precipitation.
D) self-assembly.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
31
Which is the best description of how phospholipids self-assemble?
A) spherical micelle with fatty acids on the interior of the micelle
B) spherical micelle with polar group on the interior of the micelle
C) sandwich with fatty acids forming two layers that are exposed to water
D) sandwich with polar groups forming two layers that are exposed to water
A) spherical micelle with fatty acids on the interior of the micelle
B) spherical micelle with polar group on the interior of the micelle
C) sandwich with fatty acids forming two layers that are exposed to water
D) sandwich with polar groups forming two layers that are exposed to water

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
32
What material(s) compose a cell membrane?
A) cellulose
B) membrane protein only
C) membrane protein and phospholipids
D) phospholipids only
A) cellulose
B) membrane protein only
C) membrane protein and phospholipids
D) phospholipids only

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
33
How is water transported across a cell membrane?
A) The protein aquaporin provides a hydrophilic pathway through the cell membrane.
B) Water is soluble within the cell membrane and can easily move through it.
C) Water links with the protein aquaporin, then this pair moves through the cell membrane.
D) Water moves through pores where the phospholipids do not fit tightly together in the bilayer.
A) The protein aquaporin provides a hydrophilic pathway through the cell membrane.
B) Water is soluble within the cell membrane and can easily move through it.
C) Water links with the protein aquaporin, then this pair moves through the cell membrane.
D) Water moves through pores where the phospholipids do not fit tightly together in the bilayer.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
34
Why does a solution of sodium chloride, NaCl, in water conduct electricity?
A) Pure water is a very good conductor of electricity.
B) Solid NaCl is a very good conductor of electricity, so the NaCl particles in the solution conduct electricity.
C) NaCl breaks into the ions Na+ and Cl- that may move through the solution to conduct electricity.
D) The NaCl particle forms a bond with water, thus allowing the solution to conduct electricity.
A) Pure water is a very good conductor of electricity.
B) Solid NaCl is a very good conductor of electricity, so the NaCl particles in the solution conduct electricity.
C) NaCl breaks into the ions Na+ and Cl- that may move through the solution to conduct electricity.
D) The NaCl particle forms a bond with water, thus allowing the solution to conduct electricity.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following substances is an electrolyte in water?
A) KCl
B) CH₃OH
C) sucrose
D) CH₃CH₃
A) KCl
B) CH₃OH
C) sucrose
D) CH₃CH₃

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
36
A negatively charged electrode, the cathode, and a positively charge electrode, the cathode, are placed into an aqueous solution of sodium chloride. In which direction do the Na+ ions flow?
A) toward the anode
B) toward the cathode
C) The direction of travel is not affected by the electrodes.
D) toward both electrodes
A) toward the anode
B) toward the cathode
C) The direction of travel is not affected by the electrodes.
D) toward both electrodes

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
37
Why does NaCl dissolve in water?
A) The NaCl particle is small enough to fit between water molecules
B) The partial charges on the NaCl are attracted to the partial charges on the water.
C) Water dissociates into H+ and OH- ions that can dissolve NaCl.
D) NaCl dissociates into the ions Na+ and Cl- that are highly attracted to the partial charges on the H2O molecule.
A) The NaCl particle is small enough to fit between water molecules
B) The partial charges on the NaCl are attracted to the partial charges on the water.
C) Water dissociates into H+ and OH- ions that can dissolve NaCl.
D) NaCl dissociates into the ions Na+ and Cl- that are highly attracted to the partial charges on the H2O molecule.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following pairs of solutions will form a precipitate when combined?
A) NaCl solution and NH₄NO₃ solution
B) NaCl solution and AgNO₃ solution
C) KCO₃ solution and NH₄NO₃ solution
D) AgNO₃ solution and KClO₃ solution
A) NaCl solution and NH₄NO₃ solution
B) NaCl solution and AgNO₃ solution
C) KCO₃ solution and NH₄NO₃ solution
D) AgNO₃ solution and KClO₃ solution

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
39
Select the complete ionic equation for the mixing of sodium carbonate, Na2CO3, solution and calcium nitrate, Ca(NO3)2, solution.
A) Na₂CO₃(aq) + Ca(NO₃)2(aq) CaCO₃(s) + 2NaNO₃(aq)
B) 2Na+(aq) + CO₃2-(aq) + Ca2+(aq) + 2NO₃-(aq) CaCO₃(s) + 2Na+(aq) + 2NO₃-(aq)
C) CO₃2-(aq) + Ca2+(aq) CaCO₃(s)
D) 2Na+(aq) + CO₃2-(aq) + Ca2+(aq) + 2NO₃-(aq) CaCO₃(s)
A) Na₂CO₃(aq) + Ca(NO₃)2(aq) CaCO₃(s) + 2NaNO₃(aq)
B) 2Na+(aq) + CO₃2-(aq) + Ca2+(aq) + 2NO₃-(aq) CaCO₃(s) + 2Na+(aq) + 2NO₃-(aq)
C) CO₃2-(aq) + Ca2+(aq) CaCO₃(s)
D) 2Na+(aq) + CO₃2-(aq) + Ca2+(aq) + 2NO₃-(aq) CaCO₃(s)

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
40
Select the net ionic equation for the mixing of silver nitrate, AgNO3, solution and potassium iodide, KI, solution.
A) Ag+(aq) + I-(aq) AgI(s)
B) AgNO₃(aq) + KI(aq) AgI(s) + KNO₃(aq)
C) Ag+(aq) + NO₃-(aq) + K+(aq) + I-(aq) AgI(s) + NO₃-(aq) + K+(aq)
D) Ag+(aq) + NO₃-(aq) + K+(aq) + I-(aq) AgI(s)
A) Ag+(aq) + I-(aq) AgI(s)
B) AgNO₃(aq) + KI(aq) AgI(s) + KNO₃(aq)
C) Ag+(aq) + NO₃-(aq) + K+(aq) + I-(aq) AgI(s) + NO₃-(aq) + K+(aq)
D) Ag+(aq) + NO₃-(aq) + K+(aq) + I-(aq) AgI(s)

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following substances would not be good to be present within the interior of a cell?
A) NaCl
B) NH₄NO₃
C) CaCO₃
D) KI
A) NaCl
B) NH₄NO₃
C) CaCO₃
D) KI

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
42
In a total ionic equation, where would spectator ions be found?
A) reactant side
B) product side
C) both reactant and product sides
D) neither reactant or product side
A) reactant side
B) product side
C) both reactant and product sides
D) neither reactant or product side

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
43
How do Ions move across the cell membrane?
A) Ions are soluble in the polar ends of the phospholipids.
B) Ions form cation-anion pairs that are soluble in the nonpolar interior of the bilayer.
C) Proteins called ion channels allow the ions to cross the membrane.
D) The ions squeeze between the phospholipids.
A) Ions are soluble in the polar ends of the phospholipids.
B) Ions form cation-anion pairs that are soluble in the nonpolar interior of the bilayer.
C) Proteins called ion channels allow the ions to cross the membrane.
D) The ions squeeze between the phospholipids.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
44
Nerve cells send electrical signals by pumping ions across the cell membrane. What prevents the ions from freely flowing across the membrane?
A) ion channels
B) nonpolar interior of the phospholipid bilayer
C) They bind to the polar ends of the phospholipid bilayer.
D) Water only exists on the interior of the cell.
A) ion channels
B) nonpolar interior of the phospholipid bilayer
C) They bind to the polar ends of the phospholipid bilayer.
D) Water only exists on the interior of the cell.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
45
Certain hydrocarbons exist in the liquid state because of an intermolecular attraction called ____________________.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
46
A measure of the degree of randomness or disorder within a system of atoms or molecules is ____________________.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
47
A momentary fluctuation in the electron density of a nonpolar molecule produces an ____________________ dipole.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
48
A molecule capable of dissolving in a nonpolar solvent is ____________________-soluble.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
49
____________________-soluble vitamins may eventually become toxic in the body.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
50
Phospholipids self-assemble to form a ____________________.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
51
Ions are transported across cell membranes by ____________________.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
52
What causes an instantaneous dipole in a hydrocarbon?

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
53
What factors are considered when determining whether an alcohol is soluble in water?

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
54
Explain why a triglyceride behaves like a nonpolar solvent.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
55
Why does the USDA require a higher dose of water-soluble vitamins than fat-soluble vitamins?

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
56
Describe the structure of a detergent micelle.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
57
Describe the difference between a triglyceride and phospholipid.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
58
Describe an ion channel.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck


