Deck 4: Carbon: The Element of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/64
Play
Full screen (f)
Deck 4: Carbon: The Element of Life
1
Carbon atoms are able to form which type of covalent bond?
A) single
B) double
C) triple
D) all of the above
E) none of the above
A) single
B) double
C) triple
D) all of the above
E) none of the above
D
2
Hydrocarbon molecules contain
A) carbon, hydrogen, and oxygen.
B) water and carbon.
C) hydrogen and carbon.
D) hydrogen, carbon, nitrogen, oxygen, phosphorus, and sulfur.
A) carbon, hydrogen, and oxygen.
B) water and carbon.
C) hydrogen and carbon.
D) hydrogen, carbon, nitrogen, oxygen, phosphorus, and sulfur.
C
3
Hydrocarbon molecules with at least one C=C double bond are called
A) alkenes.
B) alkynes.
C) aromatics.
D) alkanes.
A) alkenes.
B) alkynes.
C) aromatics.
D) alkanes.
A
4
Which of these represents a methyl group?
A) --C2H5
B) --CH3
C) --CH2OH
D) --C6H6
E) none of the above
A) --C2H5
B) --CH3
C) --CH2OH
D) --C6H6
E) none of the above

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
5
Different molecular structures that arise from rotating a single bond are called
A) congeners.
B) isomers.
C) enantiomers.
D) conformers.
A) congeners.
B) isomers.
C) enantiomers.
D) conformers.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
6
The conformation of ethane where hydrogen atoms overlap each other when looking along the direction of the C-C bond is called
A) eclipsed.
B) overlapping.
C) staggered.
D) coincident.
E) crowded.
A) eclipsed.
B) overlapping.
C) staggered.
D) coincident.
E) crowded.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
7
Name the hydrocarbon represented by this formula: c5H12.
A) pentane
B) propene
C) propane
D) heptane
E) pentyne
A) pentane
B) propene
C) propane
D) heptane
E) pentyne

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
8
Name the hydrocarbon represented by this formula: c3H8.
A) pentane
B) pentyne
C) propane
D) propyne
E) propene
A) pentane
B) pentyne
C) propane
D) propyne
E) propene

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
9
The hydrocarbon butane contains how many carbon atoms?
A) two
B) three
C) four
D) five
E) six
A) two
B) three
C) four
D) five
E) six

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
10
The __________ arrangement is the most stable for adjacent carbons in a hydrocarbon chain.
A) ring
B) eclipsed
C) staggered
D) bonded
E) sandwich
A) ring
B) eclipsed
C) staggered
D) bonded
E) sandwich

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
11
Carbon atoms within alkanes have a(n) ______________ geometry
A) tetrahedral
B) octahedral
C) trigonal
D) pyramidal
E) linear
A) tetrahedral
B) octahedral
C) trigonal
D) pyramidal
E) linear

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
12
Molecules having the same molecular formula but different bonding connections between atoms are called structural
A) enantiomers.
B) twins.
C) conformers.
D) isomers.
E) rotamers.
A) enantiomers.
B) twins.
C) conformers.
D) isomers.
E) rotamers.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
13
Of the structures drawn below, three are the same molecule and one is a different isomer. Choose the one that is different.
A)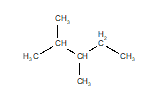
B)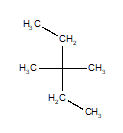
C)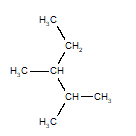
D)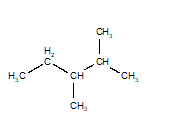
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
14
Choose the structure that represents 3-ethyloctane.
A)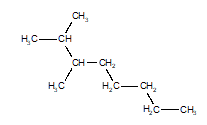
B)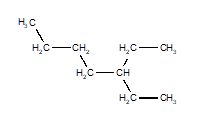
C)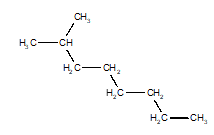
D)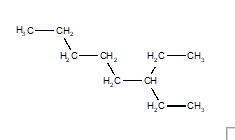
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
15
Identify the number of carbon atoms in the following molecule.
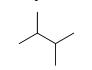
A) two
B) four
C) six
D) eight

A) two
B) four
C) six
D) eight

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
16
Identify the number of carbon atoms in the following molecule.
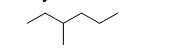
A) five
B) six
C) seven
D) eight

A) five
B) six
C) seven
D) eight

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
17
Identify the number of carbon atoms in the following molecule.
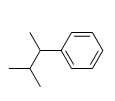
A) 10
B) 11
C) 12
D) 13

A) 10
B) 11
C) 12
D) 13

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
18
Identify the number of hydrogen atoms in the following molecule.
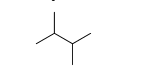
A) 14
B) 12
C) 6
D) 8

A) 14
B) 12
C) 6
D) 8

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
19
Identify the number of hydrogen atoms in the following molecule.
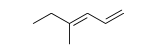
A) 11
B) 14
C) 10
D) 12

A) 11
B) 14
C) 10
D) 12

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
20
Identify the number of hydrogen atoms in the following molecule.
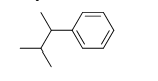
A) 11
B) 16
C) 12
D) 21

A) 11
B) 16
C) 12
D) 21

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
21
Choose the correct chemical formula for in the following molecule.
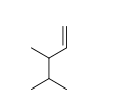
A) C7H16
B) C7H14
C) C3H8
D) C3H5
E) C7H9

A) C7H16
B) C7H14
C) C3H8
D) C3H5
E) C7H9

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
22
Choose the correct chemical formula in the following molecule. 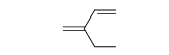
A) C6H8
B) C6H9
C) C6H10
D) C5H10
E) C5H12

A) C6H8
B) C6H9
C) C6H10
D) C5H10
E) C5H12

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
23
Choose the correct chemical formula in the following molecule.
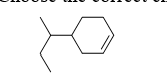
A) C10H16
B) C10H20
C) C10H12
D) C10H15
E) C10H18

A) C10H16
B) C10H20
C) C10H12
D) C10H15
E) C10H18

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
24
What is the three-dimensional structure of the ethene molecule (C2H4)?
A) planar
B) tetrahedral
C) octahedral
D) trigonal bipyramidal
E) trigonal pyramidal
A) planar
B) tetrahedral
C) octahedral
D) trigonal bipyramidal
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
25
How do double covalent bonds differ from single covalent bonds?
A) They do not allow rotation.
B) They are shorter.
C) They lead to cis and trans isomers.
D) all of the above
A) They do not allow rotation.
B) They are shorter.
C) They lead to cis and trans isomers.
D) all of the above

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
26
Another name for a dietary fat molecule is
A) triphosphate.
B) carbohydrate.
C) deoxyribonuclease.
D) triglyceride.
E) hemoglogin.
A) triphosphate.
B) carbohydrate.
C) deoxyribonuclease.
D) triglyceride.
E) hemoglogin.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
27
This is the type of fat where the hydrocarbon chains contain only C-C single bonds.
A) omega
B) saturated
C) unsaturated
D) globular
A) omega
B) saturated
C) unsaturated
D) globular

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
28
This is the type of fat where the hydrocarbon chains contain at least one C=C double bond.
A) granular
B) saturated
C) unsaturated
D) globular
A) granular
B) saturated
C) unsaturated
D) globular

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
29
Choose the characteristic of saturated fats:
A) tend to be liquid at room temperature
B) come mostly from animal sources
C) are recommended highly by nutritionists
D) all of the above
E) none of the above
A) tend to be liquid at room temperature
B) come mostly from animal sources
C) are recommended highly by nutritionists
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
30
Choose the characteristic of unsaturated fats.
A) tend to be solid at room temperature
B) come only from animal sources
C) include omega-3 fats
D) all of the above
E) none of the above
A) tend to be solid at room temperature
B) come only from animal sources
C) include omega-3 fats
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
31
The kind of fat that is considered good for the diet, slowing hardening of arteries and reducing the chance of heart attacks and strokes is
A) saturated
B) omega-3
C) unsaturated
D) trans
E) lipous
A) saturated
B) omega-3
C) unsaturated
D) trans
E) lipous

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
32
This type of fat is linked to increased risk of heart disease and has been banned by the FDA:
A) saturated
B) omega-3
C) unsaturated
D) trans
E) polyunsaturated
A) saturated
B) omega-3
C) unsaturated
D) trans
E) polyunsaturated

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
33
The process of hydrogenation converts a
A) C=C double bond to a C-C single bond.
B) triglyceride into an omega-3 fatty acid.
C) straight-chain hydrocarbon into a branched hydrocarbon.
D) saturated fat into an unsaturated fat.
A) C=C double bond to a C-C single bond.
B) triglyceride into an omega-3 fatty acid.
C) straight-chain hydrocarbon into a branched hydrocarbon.
D) saturated fat into an unsaturated fat.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
34
Where do trans fats come from?
A) consumption of too much sugar along with fatty foods produces them
B) They are converted by our bodies from unsaturated fats in our diets.
C) They are present naturally in animal fats.
D) They are a by-product of the hydrogenation process.
A) consumption of too much sugar along with fatty foods produces them
B) They are converted by our bodies from unsaturated fats in our diets.
C) They are present naturally in animal fats.
D) They are a by-product of the hydrogenation process.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
35
The hydrogenation process for oils
A) lengthens the shelf life of healthier unsaturated fats.
B) produces a solid, rather than a liquid fat source from vegetable oils.
C) involves bubbling hydrogen gas through hot oil in the presence of a metal catalyst.
D) all of the above
E) b and c only
A) lengthens the shelf life of healthier unsaturated fats.
B) produces a solid, rather than a liquid fat source from vegetable oils.
C) involves bubbling hydrogen gas through hot oil in the presence of a metal catalyst.
D) all of the above
E) b and c only

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
36
The bonding geometry around carbon atoms in a cyclohexane ring is
A) planar.
B) tetrahedral.
C) octahedral.
D) trigonal pyramidal.
A) planar.
B) tetrahedral.
C) octahedral.
D) trigonal pyramidal.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
37
The bonding geometry around carbon atoms in a benzene ring is
A) planar.
B) tetrahedral.
C) octahedral.
D) trigonal pyramidal.
A) planar.
B) tetrahedral.
C) octahedral.
D) trigonal pyramidal.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
38
Describe the molecular structure of a benzene ring.
A) six carbon atoms in the shape of a flat hexagon with three longer single bonds and three shorter double bonds
B) six carbon atoms in a ring that takes on the three-dimensional shape of a chair
C) Six carbon atoms in a ring that takes on the three-dimensional shape of a boat
D) Six carbon atoms in the shape of a flat hexagon, each C-C bond is the same length
A) six carbon atoms in the shape of a flat hexagon with three longer single bonds and three shorter double bonds
B) six carbon atoms in a ring that takes on the three-dimensional shape of a chair
C) Six carbon atoms in a ring that takes on the three-dimensional shape of a boat
D) Six carbon atoms in the shape of a flat hexagon, each C-C bond is the same length

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
39
Electrons in the double bonds of a benzene ring are
A) delocalized.
B) departmentalized.
C) energized.
D) resonant.
E) quantized.
A) delocalized.
B) departmentalized.
C) energized.
D) resonant.
E) quantized.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
40
When two or more equivalent bonding arrangements exist in a molecule, they are called
A) Lewis dot structures.
B) resonance structures.
C) isomers.
D) rotamers.
E) conformers.
A) Lewis dot structures.
B) resonance structures.
C) isomers.
D) rotamers.
E) conformers.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
41
Molecules containing a benzene ring are called
A) alkanes.
B) aromatic.
C) alkynes.
D) alkenes.
E) aliphatic.
A) alkanes.
B) aromatic.
C) alkynes.
D) alkenes.
E) aliphatic.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
42
A widely-used method for determining the structure of molecules is called
A) X-ray photography.
B) molecular electrolysis.
C) capillary electrophoresis.
D) X-ray crystallography.
E) molecular microscopy.
A) X-ray photography.
B) molecular electrolysis.
C) capillary electrophoresis.
D) X-ray crystallography.
E) molecular microscopy.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
43
The molecule that contributes to a condition known as atherosclerosis is
A) benzene.
B) cholesterol.
C) triglyceride.
D) cyclohexane.
E) carbohydrate.
A) benzene.
B) cholesterol.
C) triglyceride.
D) cyclohexane.
E) carbohydrate.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
44
The following molecule is toluene, found in paint thinners and used as an industrial solvent. Overexposure to toluene has the ability to cause severe neurological harm. Choose the correct chemical formula for toluene.
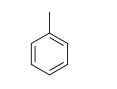
A) C7H14
B) C6H8
C) C6H6
D) C7H8
E) C7H12

A) C7H14
B) C6H8
C) C6H6
D) C7H8
E) C7H12

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
45
The following molecule is biphenyl, used as a food additive to prevent growth of molds and fungus. Choose the correct chemical formula for biphenyl.
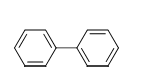
A) C12H10
B) C12H12
C) C12H20
D) C12H18
E) C12H19

A) C12H10
B) C12H12
C) C12H20
D) C12H18
E) C12H19

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
46
The following molecule is myrcene, found in lemongrass tea. It has an analgesic (pain-relieving) effect, and is likely responsible for the tea's medicinal properties. Choose the correct chemical formula for myrcene.
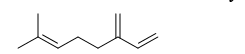
A) C8H12
B) C8H14
C) C10H16
D) C10H18
E) C10H14

A) C8H12
B) C8H14
C) C10H16
D) C10H18
E) C10H14

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
47
The following molecule is pinene, a natural compound repellent to insects and found in the resin of pine and other trees and plants. Choose the correct chemical formula for pinene.
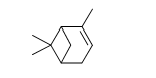
A) C8H12
B) C8H14
C) C9H12
D) C10H16
E) C10H14

A) C8H12
B) C8H14
C) C9H12
D) C10H16
E) C10H14

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
48
A(n) ____________________ fat contains only single covalent bonds between carbon atoms; a(n) ____________________ fat contains at least one double bond joining two carbon atoms.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
49
Humulene, following pictured as a line-angle drawing, is produced by the hops plant and is found to have anti-inflammatory properties. Draw the full-atom structure for humulene and write the chemical formula.
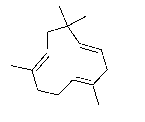


Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
50
Vitamin D3, following pictured as a line-angle drawing, is essential for healthy bones. Determine the number of carbon and hydrogen atoms in the molecule. There is also one oxygen atom present. Write the chemical formula for vitamin D3.
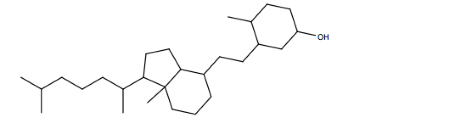


Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
51
In the __________ isomer, two hydrocarbon groups are on opposite sides of a C=C double bond.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
52
Explain why saturated fats are usually solids at room temperature, while unsaturated fats are usually liquids.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
53
The benzene molecule can be drawn in two equivalent structures with different positions for the C=C double bonds. This is called _____________.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
54
Match the chemical names to the molecular structure.
-Cis-3-hexene
A)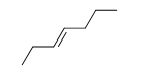
B)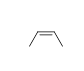
C)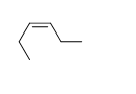
D)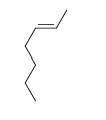
E)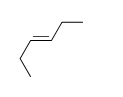 f.
f. 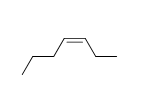
-Cis-3-hexene
A)

B)

C)

D)

E)
 f.
f. 

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
55
Match the chemical names to the molecular structure.
-Trans-3-hexene
A)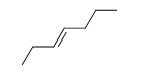
B)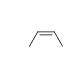
C)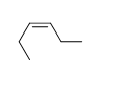
D)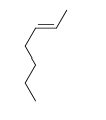
E)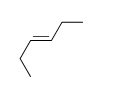 f.
f. 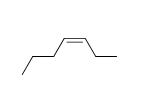
-Trans-3-hexene
A)

B)

C)

D)

E)
 f.
f. 

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
56
Match the chemical names to the molecular structure.
-Trans-2-heptene
A)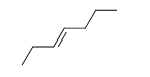
B)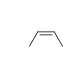
C)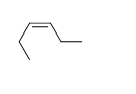
D)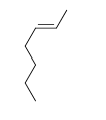
E)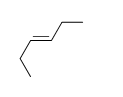 f.
f. 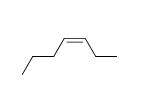
-Trans-2-heptene
A)

B)

C)

D)

E)
 f.
f. 

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
57
Match the chemical names to the molecular structure.
-Trans-3-heptene
A)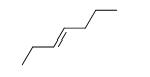
B)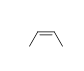
C)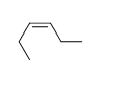
D)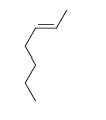
E)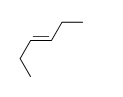 f.
f. 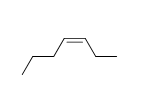
-Trans-3-heptene
A)

B)

C)

D)

E)
 f.
f. 

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
58
Match the chemical names to the molecular structure.
-Cis-3-heptene
A)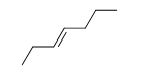
B)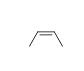
C)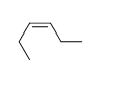
D)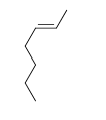
E)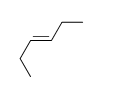 f.
f. 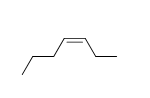
-Cis-3-heptene
A)

B)

C)

D)

E)
 f.
f. 

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
59
Match the chemical names to the molecular structure.
-Cis-2-butene
A)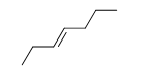
B)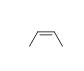
C)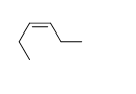
D)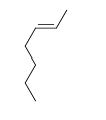
E)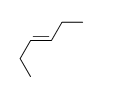 f.
f. 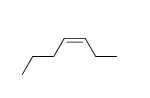
-Cis-2-butene
A)

B)

C)

D)

E)
 f.
f. 

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
60
Match each type of fat to its description.
-Saturated
A) Arises from hydrogenation of vegetable oils and is linked to increased risk of heart disease; usually found in fast foods and processed foods.
B) Contains more than one C=C double bond in the hydrocarbon chain
C) Contains only C-C single bonds, solid at room temperature. Found in butter, cheese and meat.
D) Contains at least one C=C double bond, usually a liquid at room temperature. Found in high quantities in olive oil, avocadoes and peanut oil.
E) Has a C=C double bond between carbon atoms #3 and #4, found in fish oil, flaxseed and canola oil.
-Saturated
A) Arises from hydrogenation of vegetable oils and is linked to increased risk of heart disease; usually found in fast foods and processed foods.
B) Contains more than one C=C double bond in the hydrocarbon chain
C) Contains only C-C single bonds, solid at room temperature. Found in butter, cheese and meat.
D) Contains at least one C=C double bond, usually a liquid at room temperature. Found in high quantities in olive oil, avocadoes and peanut oil.
E) Has a C=C double bond between carbon atoms #3 and #4, found in fish oil, flaxseed and canola oil.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
61
Match each type of fat to its description.
-Unsaturated
A) Arises from hydrogenation of vegetable oils and is linked to increased risk of heart disease; usually found in fast foods and processed foods.
B) Contains more than one C=C double bond in the hydrocarbon chain
C) Contains only C-C single bonds, solid at room temperature. Found in butter, cheese and meat.
D) Contains at least one C=C double bond, usually a liquid at room temperature. Found in high quantities in olive oil, avocadoes and peanut oil.
E) Has a C=C double bond between carbon atoms #3 and #5, found in fish oil, flaxseed and canola oil.
-Unsaturated
A) Arises from hydrogenation of vegetable oils and is linked to increased risk of heart disease; usually found in fast foods and processed foods.
B) Contains more than one C=C double bond in the hydrocarbon chain
C) Contains only C-C single bonds, solid at room temperature. Found in butter, cheese and meat.
D) Contains at least one C=C double bond, usually a liquid at room temperature. Found in high quantities in olive oil, avocadoes and peanut oil.
E) Has a C=C double bond between carbon atoms #3 and #5, found in fish oil, flaxseed and canola oil.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
62
Match each type of fat to its description.
-Omega-3
A) Arises from hydrogenation of vegetable oils and is linked to increased risk of heart disease; usually found in fast foods and processed foods.
B) Contains more than one C=C double bond in the hydrocarbon chain
C) Contains only C-C single bonds, solid at room temperature. Found in butter, cheese and meat.
D) Contains at least one C=C double bond, usually a liquid at room temperature. Found in high quantities in olive oil, avocadoes and peanut oil.
E) Has a C=C double bond between carbon atoms #3 and #6, found in fish oil, flaxseed and canola oil.
-Omega-3
A) Arises from hydrogenation of vegetable oils and is linked to increased risk of heart disease; usually found in fast foods and processed foods.
B) Contains more than one C=C double bond in the hydrocarbon chain
C) Contains only C-C single bonds, solid at room temperature. Found in butter, cheese and meat.
D) Contains at least one C=C double bond, usually a liquid at room temperature. Found in high quantities in olive oil, avocadoes and peanut oil.
E) Has a C=C double bond between carbon atoms #3 and #6, found in fish oil, flaxseed and canola oil.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
63
Match each type of fat to its description.
-Trans
A) Arises from hydrogenation of vegetable oils and is linked to increased risk of heart disease; usually found in fast foods and processed foods.
B) Contains more than one C=C double bond in the hydrocarbon chain
C) Contains only C-C single bonds, solid at room temperature. Found in butter, cheese and meat.
D) Contains at least one C=C double bond, usually a liquid at room temperature. Found in high quantities in olive oil, avocadoes and peanut oil.
E) Has a C=C double bond between carbon atoms #3 and #7, found in fish oil, flaxseed and canola oil.
-Trans
A) Arises from hydrogenation of vegetable oils and is linked to increased risk of heart disease; usually found in fast foods and processed foods.
B) Contains more than one C=C double bond in the hydrocarbon chain
C) Contains only C-C single bonds, solid at room temperature. Found in butter, cheese and meat.
D) Contains at least one C=C double bond, usually a liquid at room temperature. Found in high quantities in olive oil, avocadoes and peanut oil.
E) Has a C=C double bond between carbon atoms #3 and #7, found in fish oil, flaxseed and canola oil.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck
64
Match each type of fat to its description.
-Polyunsaturated
A) Arises from hydrogenation of vegetable oils and is linked to increased risk of heart disease; usually found in fast foods and processed foods.
B) Contains more than one C=C double bond in the hydrocarbon chain
C) Contains only C-C single bonds, solid at room temperature. Found in butter, cheese and meat.
D) Contains at least one C=C double bond, usually a liquid at room temperature. Found in high quantities in olive oil, avocadoes and peanut oil.
E) Has a C=C double bond between carbon atoms #3 and #8, found in fish oil, flaxseed and canola oil.
-Polyunsaturated
A) Arises from hydrogenation of vegetable oils and is linked to increased risk of heart disease; usually found in fast foods and processed foods.
B) Contains more than one C=C double bond in the hydrocarbon chain
C) Contains only C-C single bonds, solid at room temperature. Found in butter, cheese and meat.
D) Contains at least one C=C double bond, usually a liquid at room temperature. Found in high quantities in olive oil, avocadoes and peanut oil.
E) Has a C=C double bond between carbon atoms #3 and #8, found in fish oil, flaxseed and canola oil.

Unlock Deck
Unlock for access to all 64 flashcards in this deck.
Unlock Deck
k this deck


