Deck 8: Organic Chemistry and Polymers
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/123
Play
Full screen (f)
Deck 8: Organic Chemistry and Polymers
1
What is the source material for most polymers?
A)Biodegradable plant matter
B)Petroleum
C)Plastics
D)Clothing, shopping bags, and disposable packaging
A)Biodegradable plant matter
B)Petroleum
C)Plastics
D)Clothing, shopping bags, and disposable packaging
B
2
How much polymer waste has ended up in landfills?
A)2.1 million tons
B)21 million tons
C)3.0 million tons
D)30 million tons
A)2.1 million tons
B)21 million tons
C)3.0 million tons
D)30 million tons
D
3
By how much can power plants that burn garbage for energy reduce the amount of space that would be taken up by that garbage in a landfill?
A)12.3%
B)7%
C)90%
D)None, because this waste does not end up in landfills.
A)12.3%
B)7%
C)90%
D)None, because this waste does not end up in landfills.
C
4
What is wrong with burning polymers after their use to make energy?
A)They are expensive materials, and burning them is a waste of money.
B)They can emit toxic substances if burned incorrectly.
C)Most polymers do not burn.
D)There is no problem with the burning of polymers.
A)They are expensive materials, and burning them is a waste of money.
B)They can emit toxic substances if burned incorrectly.
C)Most polymers do not burn.
D)There is no problem with the burning of polymers.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
5
How does an immunoassay function?
A)By having a small molecule fit into a larger one
B)By having a small molecule deform as it is fitted into a larger one
C)By having a large molecule fit into a smaller one
D)By having a large molecule deform as it is fitted into a smaller one
A)By having a small molecule fit into a larger one
B)By having a small molecule deform as it is fitted into a larger one
C)By having a large molecule fit into a smaller one
D)By having a large molecule deform as it is fitted into a smaller one

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
6
Why do covalent bonds form between two atoms?
A)Because the polarity difference between the two is large enough for one atom to strip an electron from the other
B)Because the polarity difference between the two is not large enough for one atom to strip an electron from the other
C)Because the electronegativity difference between the two is large enough for one atom to strip an electron from the other
D)Because the electronegativity difference between the two is not large enough for one atom to strip an electron from the other
A)Because the polarity difference between the two is large enough for one atom to strip an electron from the other
B)Because the polarity difference between the two is not large enough for one atom to strip an electron from the other
C)Because the electronegativity difference between the two is large enough for one atom to strip an electron from the other
D)Because the electronegativity difference between the two is not large enough for one atom to strip an electron from the other

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is a tenet of valence bond theory?
A)Electrons must not be shared.
B)Orbitals must overlap.
C)Orbitals must not overlap.
D)Electrons must remain localized.
A)Electrons must not be shared.
B)Orbitals must overlap.
C)Orbitals must not overlap.
D)Electrons must remain localized.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
8
When p orbitals overlap to form a single bond, what part of the orbital must be overlapped?
A)The end
B)The side
C)The middle
D)Both ends
A)The end
B)The side
C)The middle
D)Both ends

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
9
What is one of the major uses of organic chemistry today?
A)The production of illegal drugs
B)The synthesis of new and different plastics
C)The discovery and synthesis of new pharmaceuticals
D)The production and synthesis of new mineral compounds
A)The production of illegal drugs
B)The synthesis of new and different plastics
C)The discovery and synthesis of new pharmaceuticals
D)The production and synthesis of new mineral compounds

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
10
How does Lewis theory explain the loss or gain of electrons in an ionic bond?
A)Atoms are attempting to gain electrons.
B)Atoms are attempting to lose electrons.
C)Atoms are attempting to share valence electrons.
D)Atoms are attempting to achieve a noble gas electron configuration.
A)Atoms are attempting to gain electrons.
B)Atoms are attempting to lose electrons.
C)Atoms are attempting to share valence electrons.
D)Atoms are attempting to achieve a noble gas electron configuration.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
11
What do the valence shells of all the noble gas elements have in common?
A)A large size for its row on the periodic table
B)A high ability to lose electrons
C)A filled electron shell
D)A high affinity for further electrons
A)A large size for its row on the periodic table
B)A high ability to lose electrons
C)A filled electron shell
D)A high affinity for further electrons

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
12
What is the octet rule?
A)Elements form bonds to become isoelectronic with the noble gases.
B)Elements form bonds to avoid becoming isoelectronic with the noble gases.
C)Elements do not form bonds to become isoelectronic with the noble gases.
D)Elements do not form bonds to avoid becoming isoelectronic with the noble gases.
A)Elements form bonds to become isoelectronic with the noble gases.
B)Elements form bonds to avoid becoming isoelectronic with the noble gases.
C)Elements do not form bonds to become isoelectronic with the noble gases.
D)Elements do not form bonds to avoid becoming isoelectronic with the noble gases.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
13
The octet rule is quite different from the "rule of eight."

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
14
Which is the correct Lewis dot structure for He?
A)He:
B)H:
C)He.
D)H.
A)He:
B)H:
C)He.
D)H.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
15
What is the proper Lewis dot structure for Ca?
A)Ca:
B).Ca.
C).Ca
D)Ca
A)Ca:
B).Ca.
C).Ca
D)Ca

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
16
The correct Lewis dot structure for potassium is what?
A).K.
B).K
C).Pt
D).Pt.
A).K.
B).K
C).Pt
D).Pt.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
17
What is the correct Lewis dot structure for Se?
A)
B)
C)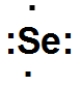
D)
A)

B)

C)
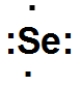
D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is an alkane?
A)CH3CH2CHCH2
B)CH3CHCHCH3
C)CH2CHCH3
D)CH3CH2CH2CH3
A)CH3CH2CHCH2
B)CH3CHCHCH3
C)CH2CHCH3
D)CH3CH2CH2CH3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
19
How many bonds does carbon normally form?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
20
Carbon can only bond to four other atoms.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
21
Does use of the rule for organic structures, "Carbon atoms bond together to form a chain," mean that organic structures will always have two end-carbon atoms?
A)Yes, always.
B)No, never.
C)Yes, but only when the compounds are alkanes.
D)Yes, if the compounds are alkanes, and are not cyclic alkanes.
A)Yes, always.
B)No, never.
C)Yes, but only when the compounds are alkanes.
D)Yes, if the compounds are alkanes, and are not cyclic alkanes.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
22
What is the formula for an alkane with ten carbons, decane?
A)C10H20
B)C10H22
C)C10H24
D)None of the above.
A)C10H20
B)C10H22
C)C10H24
D)None of the above.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
23
Which is the correct Lewis dot structure for chlorine?
A)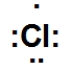
B)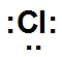
C)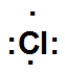
D)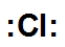
A)
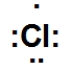
B)
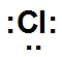
C)
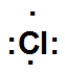
D)
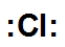

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
24
When calcium and chlorine react, they form an ionic compound. What is its Lewis dot structure?
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
25
Lewis structures with single lines indicate what kind of bonding?
A)Covalent, 2-electron bonding
B)Ionic, 2-electron bonding
C)Covalent, 4-electron bonding
D)Ionic, 4-electron bonding
A)Covalent, 2-electron bonding
B)Ionic, 2-electron bonding
C)Covalent, 4-electron bonding
D)Ionic, 4-electron bonding

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
26
What is the correct Lewis structure for ethyne, C2H2, also known by the common name acetylene?
A)H-H-C-C
B)H-C-C-H
C)H-C=C-H
D)H-C≡C-H
A)H-H-C-C
B)H-C-C-H
C)H-C=C-H
D)H-C≡C-H

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
27
The simplest covalently bonded molecule is hydrogen. Which is the correct Lewis structure?
A)H2
B)H.H
C)H=H
D)H-H
A)H2
B)H.H
C)H=H
D)H-H

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
28
Which is the best Lewis structure for methane?
A)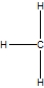
B)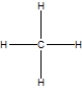
C)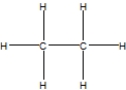
D)None of the above.
A)

B)

C)

D)None of the above.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
29
Water is best represented by which Lewis structure?
A)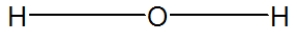
B)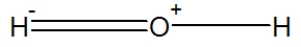
C)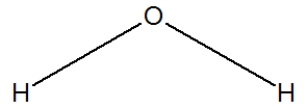
D)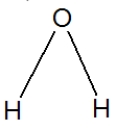
A)
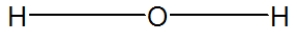
B)
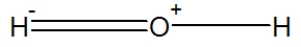
C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
30
How is ethane best represented by a Lewis structure?
A)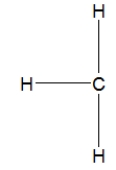
B)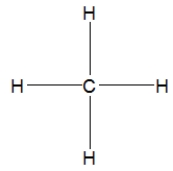
C)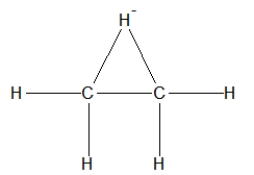
D)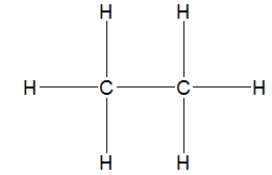
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
31
Phosphine, PH3, is structurally much like ammonia. Which of the following is the best Lewis structure of phosphine?
A)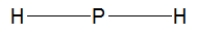
B)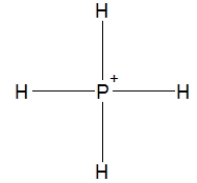
C)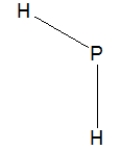
D)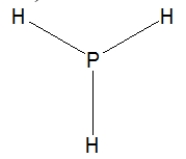
A)
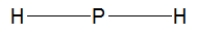
B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
32
Hydrogen sulfide is considered the "rotten egg" gas, and has a structure much like water. Which of the following is its Lewis structure?
A)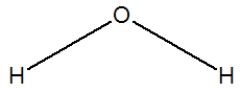
B)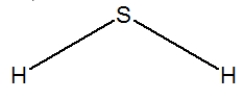
C)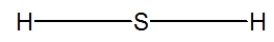
D)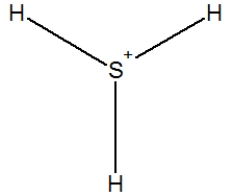
A)

B)

C)
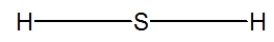
D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
33
What is wrong with the Lewis structure He == He?
A)Helium does not bond to another helium.
B)Helium only forms a single bond to a second helium.
C)Helium needs more electrons to form the bond.
D)Nothing, the bonding is correct.
A)Helium does not bond to another helium.
B)Helium only forms a single bond to a second helium.
C)Helium needs more electrons to form the bond.
D)Nothing, the bonding is correct.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
34
What does the double line, which looks much like an equals sign, represent in Lewis structures?
A)A 2-electron single bond
B)A 4-electron double bond
C)A 2-electron double bond
D)A 4-electron single bond
A)A 2-electron single bond
B)A 4-electron double bond
C)A 2-electron double bond
D)A 4-electron single bond

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following choices is the best Lewis structure for ammonia?
A)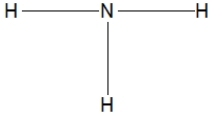
B)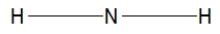
C)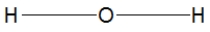
D)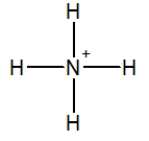
A)

B)
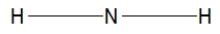
C)
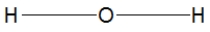
D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
36
Propane, C3H8, is best represented by which Lewis structure?
A)
B)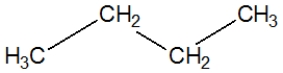
C)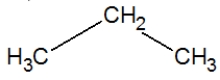
D)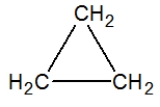
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
37
Boron trifluoride, BF3, is a corrosive material with a Lewis structure that shows boron to be two electrons short of a full octet. Which is the correct structure for BF3?
A)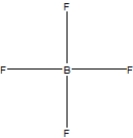
B)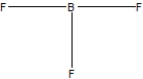
C)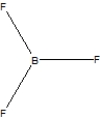
D)None of the above.
A)

B)

C)

D)None of the above.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
38
What is the correct Lewis structure for methyl chloride, CClH3?
A)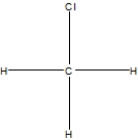
B)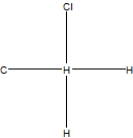
C)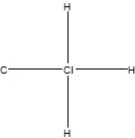
D)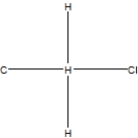
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
39
Bromine in the gaseous state is Br2 and has a simple Lewis structure. What type of bonding will it have?
A)A double, ionic bond
B)A single, ionic bond
C)A double, covalent bond
D)A single, covalent bond
A)A double, ionic bond
B)A single, ionic bond
C)A double, covalent bond
D)A single, covalent bond

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
40
What is wrong with this structure for gaseous hydrogen chloride: H - Cl?
A)The lone pair electrons of hydrogen are not shown here.
B)There are lone pair electrons on the chlorine which are not shown here.
C)The bond between the two atoms should be a double bond.
D)Nothing, the structure is correct.
A)The lone pair electrons of hydrogen are not shown here.
B)There are lone pair electrons on the chlorine which are not shown here.
C)The bond between the two atoms should be a double bond.
D)Nothing, the structure is correct.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
41
Draw for yourself the Lewis structure of ethane, C2H4, also called ethylene. How many double bonds does it have?
A)None
B)1
C)2
D)3
A)None
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
42
Which is the name of the following compound, shown as its Lewis structure?
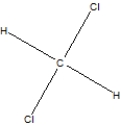
A)Chloromethane
B)Dichloromethane
C)Chloroethane
D)Dichloroethane

A)Chloromethane
B)Dichloromethane
C)Chloroethane
D)Dichloroethane

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
43
After drawing the Lewis structure for the molecule ethyne, C2H2, also known by the common name acetylene, answer this: How many double bonds does it contain?
A)None
B)1
C)2
D)3
A)None
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
44
Which is the correct Lewis structure for methanol, CH4O, also known by the common name wood alcohol?
A)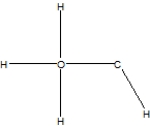
B)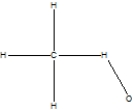
C)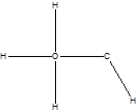
D)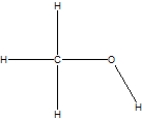
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
45
What is the best Lewis structure representation of formaldehyde, CH2O?
A)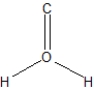
B)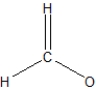
C)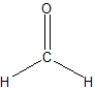
D)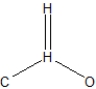
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
46
How many carbon atoms must be in the simplest alkene?
A)0
B)1
C)2
D)3
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
47
How many bonds does each carbon atom in an alkene normally have?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
48
The molecular formula for the simplest alkyne is C2H4.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
49
How many carbon atoms should be in the molecule 2-pentyne?
A)2
B)3
C)4
D)5
A)2
B)3
C)4
D)5

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
50
What elements other than carbon must be in an alkene or an alkyne?
A)Hydrogen
B)Hydrogen and oxygen
C)Hydrogen and nitrogen
D)Hydrogen, oxygen, and nitrogen
A)Hydrogen
B)Hydrogen and oxygen
C)Hydrogen and nitrogen
D)Hydrogen, oxygen, and nitrogen

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
51
Draw the cyclic, organic molecule benzene, C6H6. How many resonance structures can exist for it?
A)2
B)1
C)3
D)None
A)2
B)1
C)3
D)None

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following represent the best resonance structures for SO2?
A)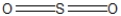
B)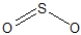
C)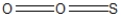
D)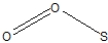
A)
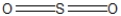
B)

C)
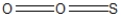
D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
53
Ozone, O3, can be drawn with more than one resonance structure. Which of the following are correct?
A)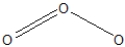
B)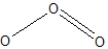
C)Both of the above.
D)None of the above.
A)

B)

C)Both of the above.
D)None of the above.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following would be correct Lewis resonance structure for the nitrite ion, NO2- ?
A)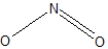
B)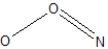
C)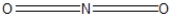
D)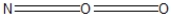
A)

B)

C)
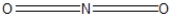
D)
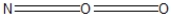

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
55
The name 2-butylpropane is incorrect for a branched alkane, but the correct name can be deduced from it. What is it?
A)1-methylhexane
B)1-methypentane
C)2-methylhexane
D)2-pentylmethane
A)1-methylhexane
B)1-methypentane
C)2-methylhexane
D)2-pentylmethane

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
56
What is the correct molecular formula for methylpropane?
A)C3H8
B)C4H8
C)C3H10
D)C4H10
A)C3H8
B)C4H8
C)C3H10
D)C4H10

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
57
How many linear or branched alkanes can be constructed from the formula C4H10?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
58
What is the difference between 1-methylpentane and 2-methylpentane?
A)The position of the methyl group
B)The length of the pentane chain
C)The end from which the methyl group position is counted
D)There is no "1-methylpentane"; it is hexane. Thus, the two compounds are isomers.
A)The position of the methyl group
B)The length of the pentane chain
C)The end from which the methyl group position is counted
D)There is no "1-methylpentane"; it is hexane. Thus, the two compounds are isomers.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
59
What does valence shell electron pair repulsion theory help determine?
A)Atomic geometry
B)Molecular geometry
C)Molecular size
D)Atomic size
A)Atomic geometry
B)Molecular geometry
C)Molecular size
D)Atomic size

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
60
What is the shape of the water molecule?
A)Trigonal, planar
B)Planar, linear
C)Planar, bent
D)Trigonal, bent
A)Trigonal, planar
B)Planar, linear
C)Planar, bent
D)Trigonal, bent

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
61
What is the geometry of the very simple molecule carbon monoxide?
A)Linear
B)Bent, planar
C)Square planar
D)Horizontal
A)Linear
B)Bent, planar
C)Square planar
D)Horizontal

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
62
What shape does the ammonia molecule have?
A)Trigonal planar
B)Square planar
C)Trigonal pyramidal
D)Square pyramidal
A)Trigonal planar
B)Square planar
C)Trigonal pyramidal
D)Square pyramidal

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
63
Any molecule that has a central atom with no lone pairs, and four other, equivalent atoms connected to it, has what shape?
A)Square
B)Triangular
C)Tetrahedral
D)Flat
A)Square
B)Triangular
C)Tetrahedral
D)Flat

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
64
The four atoms of acetylene, C2H2, known more formally as ethyne, have what molecular shape?
A)Tetrahedral
B)Square
C)Bent
D)Linear
A)Tetrahedral
B)Square
C)Bent
D)Linear

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
65
After drawing the Lewis structure for phosphine, PH3, which should be structurally similar to ammonia, what shape should the molecule have?
A)A tetrahedron
B)A triangular pyramid
C)A square plane
D)A square pyramid
A)A tetrahedron
B)A triangular pyramid
C)A square plane
D)A square pyramid

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
66
How is aniline best described?
A)As an alkene
B)As a cyclic alkane
C)As a cyclic alkyne
D)As an aromatic compound based on benzene
A)As an alkene
B)As a cyclic alkane
C)As a cyclic alkyne
D)As an aromatic compound based on benzene

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
67
What is one important use of phenol in forensic chemistry?
A)As an inhalant
B)As a paint solvent
C)As an ingredient in some fingerprint-developer formulations
D)As an accelerant
A)As an inhalant
B)As a paint solvent
C)As an ingredient in some fingerprint-developer formulations
D)As an accelerant

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
68
What does molecular polarity affect?
A)Solvent interaction
B)Melting point and boiling point
C)Both of the above
D)None of the above
A)Solvent interaction
B)Melting point and boiling point
C)Both of the above
D)None of the above

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
69
As you move from the top to the bottom of the periodic table, what happens to the electronegativity of the elements?
A)It increases.
B)It decreases.
C)Nothing, it remains relatively equal.
D)More information is needed to tell.
A)It increases.
B)It decreases.
C)Nothing, it remains relatively equal.
D)More information is needed to tell.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
70
What trend do the noble gases display in relation to electronegativity?
A)Electronegativity decreases as one goes down the noble gas column.
B)Electronegativity increases as one goes down the noble gas column.
C)The noble gases are all considered to have zero electronegativity.
D)For this column, there is no trend in electronegativity differences.
A)Electronegativity decreases as one goes down the noble gas column.
B)Electronegativity increases as one goes down the noble gas column.
C)The noble gases are all considered to have zero electronegativity.
D)For this column, there is no trend in electronegativity differences.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
71
How is the S-H bond categorized?
A)Polar covalent
B)Nonpolar covalent
C)Covalent
D)Ionic
A)Polar covalent
B)Nonpolar covalent
C)Covalent
D)Ionic

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
72
What best describes the Cl-Cl bond?
A)Non-polar covalent
B)Covalent
C)Polar covalent
D)Ionic
A)Non-polar covalent
B)Covalent
C)Polar covalent
D)Ionic

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
73
How is the N-Br bond described?
A)Pure covalent
B)Polar covalent
C)Nonpolar covalent
D)Ionic
A)Pure covalent
B)Polar covalent
C)Nonpolar covalent
D)Ionic

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
74
What is the electronegativity difference in the C-O bond?
A)1.0
B)2.5
C)3.5
D)0
A)1.0
B)2.5
C)3.5
D)0

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
75
How great is the electronegativity difference in the average C-F bond?
A)0.5
B)1.0
C)1.5
D)2.0
A)0.5
B)1.0
C)1.5
D)2.0

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
76
Many organic compounds have numerous C-H bonds. What is the average difference in electronegativity in one of them?
A)4.5
B)2.5
C)2.1
D)0.4
A)4.5
B)2.5
C)2.1
D)0.4

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
77
The compound H2Se smells absolutely horrible (worse than rotten eggs!). What is the electronegativity difference in one of the H-Se bonds?
A)2.4
B)2.1
C)0.4
D)0.3
A)2.4
B)2.1
C)0.4
D)0.3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
78
How can you describe the molecule CO2 in terms of polarity?
A)A polar molecule made from polar covalent bonds
B)A non-polar molecule made from polar covalent bonds
C)A non-polar molecule made from nonpolar covalent bonds
D)A polar molecule made from nonpolar covalent bonds
A)A polar molecule made from polar covalent bonds
B)A non-polar molecule made from polar covalent bonds
C)A non-polar molecule made from nonpolar covalent bonds
D)A polar molecule made from nonpolar covalent bonds

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
79
In terms of polarity, what best describes the molecule NH3?
A)A non-polar molecule composed of polar covalent bonds
B)A polar molecule composed of nonpolar covalent bonds
C)A polar molecule composed of polar covalent bonds
D)A non-polar molecule composed of nonpolar covalent bonds
A)A non-polar molecule composed of polar covalent bonds
B)A polar molecule composed of nonpolar covalent bonds
C)A polar molecule composed of polar covalent bonds
D)A non-polar molecule composed of nonpolar covalent bonds

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
80
How is the polarity of the molecule carbon monoxide best described?
A)A polar molecule made from a polar covalent bond
B)A non-polar molecule made from a polar covalent bond
C)A polar molecule made from a nonpolar covalent bond
D)None of the above
A)A polar molecule made from a polar covalent bond
B)A non-polar molecule made from a polar covalent bond
C)A polar molecule made from a nonpolar covalent bond
D)None of the above

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck


