Deck 15: Equilibria: How Far Do Reactions Go
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/17
Play
Full screen (f)
Deck 15: Equilibria: How Far Do Reactions Go
1
What is the equilibrium constant for the reaction A ⇌ 2B if the equilibrium concentrations are [A] = 4.27 10-2 M, [B] = 1.41 10-2 M?
A) 4.66 10-4
B) 7.32 10-3
C) 4.66 10-3
D) 2.86 10-1
E) 4.66
A) 4.66 10-4
B) 7.32 10-3
C) 4.66 10-3
D) 2.86 10-1
E) 4.66
C
2
Consider the equilibrium reaction N2 + O2 ⇌ 2NO. The equilibrium constant for this reaction is represented by which one of the following?
A)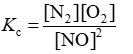
B)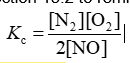
C)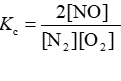
D)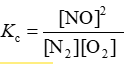
E)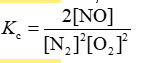
A)
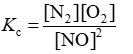
B)
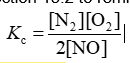
C)
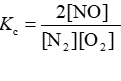
D)
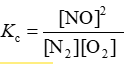
E)
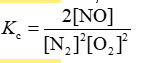
D
3
What is the equilibrium constant for the reaction A + 2B ⇌ 3C if the equilibrium concentrations are [A] = 0.413 M, [B] = 0.414 M, [C] = 0.228 M
A) 6.34 10-2
B) 5.43 10-5
C) 7.67 10-7
D) 8.52 10-1
E) 4.91 10-3
A) 6.34 10-2
B) 5.43 10-5
C) 7.67 10-7
D) 8.52 10-1
E) 4.91 10-3
E
4
Consider the reaction N2 + O2 ⇌ 2NO. At 1800 C, this reaction has an equilibrium constant of 1.2 10-4. During the course of the reaction, a sample of the reaction mixture is taken, and the concentrations of each component are found to be as follows: [N2] = 0.055 mol L-1, [O2] = 0.081 mol L-1, and [NO] = 0.003 mol L-1. In which direction must the reaction proceed for it to reach equilibrium?
A) The reaction must proceed from right to left.
B) The reaction must proceed from left to right.
C) The reaction is already at equilibrium.
A) The reaction must proceed from right to left.
B) The reaction must proceed from left to right.
C) The reaction is already at equilibrium.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the reaction 3O2 ⇌ 2O3 for which H of the forward reaction is +280 kJ mol-1. If we increase the temperature when the reaction mixture is at equilibrium, what can we expect to happen?
A) We cannot predict what will happen.
B) The equilibrium reaction will shift to the left.
C) The equilibrium reaction will shift to the right.
D) The reaction mixture will be unaltered by the increase in temperature.
A) We cannot predict what will happen.
B) The equilibrium reaction will shift to the left.
C) The equilibrium reaction will shift to the right.
D) The reaction mixture will be unaltered by the increase in temperature.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
6
Chlorine reacts with sulfur dioxide to produce thionyl chloride according to the following equilibrium reaction: SO2 + Cl2 ⇌ SO2Cl2. The forward reaction is exothermic.
What will be the consequences of adding more Cl2 to the reaction when the reaction is at equilibrium?
A) The temperature of the reaction mixture will rise.
B) The temperature of the reaction mixture will stay the same.
C) The temperature of the reaction mixture will fall.
What will be the consequences of adding more Cl2 to the reaction when the reaction is at equilibrium?
A) The temperature of the reaction mixture will rise.
B) The temperature of the reaction mixture will stay the same.
C) The temperature of the reaction mixture will fall.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
7
In the reaction H2 + I2 ⇌ 2HI, the equilibrium constant at 400C is 500. Which of the following statements are true when the reaction is at equilibrium? Select any that apply.
A) The reaction lies almost completely to the left.
B) The reaction lies almost completely to the right.
C) The Gibbs free energy change for the reaction is positive.
D) Neither the forward nor the back reactions are occurring.
E) The Gibbs free energy change for the reaction is negative.
A) The reaction lies almost completely to the left.
B) The reaction lies almost completely to the right.
C) The Gibbs free energy change for the reaction is positive.
D) Neither the forward nor the back reactions are occurring.
E) The Gibbs free energy change for the reaction is negative.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
8
The equilibrium constant for the equilibrium reaction N2 + 3H2 ⇌ 2NH3 is 70 at 500C. If 0.56 mol of NH3, 0.42 mol of N2 and 0.67 mol H2 are injected into a flask, how will the reagents behave? Select all responses that apply.
A) The reaction will proceed to the right.
B) The overall concentration of N2 present will increase.
C) The reaction will proceed to the left.
D) The reaction mixture will stay as it is.
E) The overall concentration of NH3 present will increase.
A) The reaction will proceed to the right.
B) The overall concentration of N2 present will increase.
C) The reaction will proceed to the left.
D) The reaction mixture will stay as it is.
E) The overall concentration of NH3 present will increase.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
9
If the reaction N2O4 ⇌ 2NO2 is at equilibrium and more N2O4 is fed into the reaction mixture, what will happen?
A) The equilibrium reaction will shift to the left.
B) The concentration of NO2 in the reaction mixture will have increased once equilibrium is re-established, but the concentration of N2O4 will remain the same.
C) The concentration of N2O4 in the reaction mixture will increase once equilibrium is re-established, but the concentration of NO2 will remain the same.
D) Equilibrium will not be perturbed.
E) and NO2 will have increased.
A) The equilibrium reaction will shift to the left.
B) The concentration of NO2 in the reaction mixture will have increased once equilibrium is re-established, but the concentration of N2O4 will remain the same.
C) The concentration of N2O4 in the reaction mixture will increase once equilibrium is re-established, but the concentration of NO2 will remain the same.
D) Equilibrium will not be perturbed.
E) and NO2 will have increased.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
10
What is the equilibrium constant for the reaction A + B ⇌ 2C if the equilibrium concentrations are: [A] 0.419 M, [B] 0.329 M, [C] 97.2 mM?
A) 0.123 M
B) 0.685
C) 1.47
D) 68537
E) 0.0685
A) 0.123 M
B) 0.685
C) 1.47
D) 68537
E) 0.0685

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
11
Consider the following equilibrium reaction:
CH4 + H2O ⇌ CO + 3H2.
At 298 K, K = 9.4 10-1
Which of the following statements in relation to this reaction are true? Select all that apply.
A) Increasing the pressure favours the forward reaction.
B) Increasing the concentration of CO favours the back reaction.
C) Decreasing the concentration of CH4 favours the back reaction.
D) The reaction is spontaneous.
E) At equilibrium, the reaction lies to the left: the reactants are favoured.
CH4 + H2O ⇌ CO + 3H2.
At 298 K, K = 9.4 10-1
Which of the following statements in relation to this reaction are true? Select all that apply.
A) Increasing the pressure favours the forward reaction.
B) Increasing the concentration of CO favours the back reaction.
C) Decreasing the concentration of CH4 favours the back reaction.
D) The reaction is spontaneous.
E) At equilibrium, the reaction lies to the left: the reactants are favoured.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements about equilibrium reactions are true? Select all that apply.
A) The equilibrium constant for a given reaction differs according to the temperature of the system.
B) The equilibrium constant for a given reaction at a particular temperature is the same, regardless of the concentrations of the components of the reaction mixture when they are first mixed.
C) At equilibrium, the concentration of reactants and products is equal.
D) When a reaction is at equilibrium, the value of the equilibrium constant is larger than the value of the reaction quotient.
E) At equilibrium, the rates of the forward and back reactions are equal.
A) The equilibrium constant for a given reaction differs according to the temperature of the system.
B) The equilibrium constant for a given reaction at a particular temperature is the same, regardless of the concentrations of the components of the reaction mixture when they are first mixed.
C) At equilibrium, the concentration of reactants and products is equal.
D) When a reaction is at equilibrium, the value of the equilibrium constant is larger than the value of the reaction quotient.
E) At equilibrium, the rates of the forward and back reactions are equal.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
13
A large negative G for a reaction suggests which one of the following?
A) The reaction quotient and the equilibrium constant are equal.
B) The reaction is far from equilibrium.
C) There is a lot more product than reactant present.
D) H for the reaction is large and positive.
A) The reaction quotient and the equilibrium constant are equal.
B) The reaction is far from equilibrium.
C) There is a lot more product than reactant present.
D) H for the reaction is large and positive.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following phrases correctly complete the sentence "When G = 0 for a chemical reaction, …". Select all that apply.
A) …the reaction is about to start.
B) …the reaction is at equilibrium.
C) ...H is equal to TS.
D) …the reaction is spontaneous.
A) …the reaction is about to start.
B) …the reaction is at equilibrium.
C) ...H is equal to TS.
D) …the reaction is spontaneous.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
15
Consider the equilibrium reaction A + 2B ⇌ 3C. When the reaction mixture is sampled prior to equilibrium being reached, it is determined that the reaction must proceed to the left for equilibrium to be reached. When the reaction mixture is sampled at equilibrium, the following concentrations are measured: [A] 0.267 mol L-1, [B] 0.336 mol L-1 , [C] 0.173 mol L-1. What were the most likely concentrations of A, B, and C when the reaction mixture was sampled prior to equilibrium?
A) [A] = 0.124 mol L-1; [B] = 0.225 mol L-1; [C] = 0.334 mol L-1
B) [A] = 0.334 mol L-1; [B] = 0.225 mol L-1; [C] = 0.124 mol L-1
C) [A] = 0.225 mol L-1; [B] = 0.334 mol L-1; [C] = 0.124 mol L-1
A) [A] = 0.124 mol L-1; [B] = 0.225 mol L-1; [C] = 0.334 mol L-1
B) [A] = 0.334 mol L-1; [B] = 0.225 mol L-1; [C] = 0.124 mol L-1
C) [A] = 0.225 mol L-1; [B] = 0.334 mol L-1; [C] = 0.124 mol L-1

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
16
The binding of a potential substrate to an enzyme has a large dissociation constant, Kd. Therefore the enzyme and substrate show a strong affinity for each other.

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck
17
Nitrogen and oxygen react together according to the following equilibrium reaction:
N2(g) + O2(g) ⇌ 2 NO(g)
If the equilibrium constant for this reaction, Kp, is 2 10-37 at a given temperature what is the partial pressure of NO at equilibrium?
A) 2.7 10-22 kPa
B) 90.9 1018 kPa
C) 1.8 10-17 kPa
D) 3.3 10-34 kPa
N2(g) + O2(g) ⇌ 2 NO(g)
If the equilibrium constant for this reaction, Kp, is 2 10-37 at a given temperature what is the partial pressure of NO at equilibrium?
A) 2.7 10-22 kPa
B) 90.9 1018 kPa
C) 1.8 10-17 kPa
D) 3.3 10-34 kPa

Unlock Deck
Unlock for access to all 17 flashcards in this deck.
Unlock Deck
k this deck


